Abstract
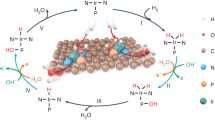
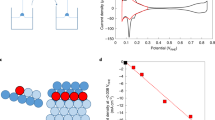
The development of hydrogen-based energy sources as viable alternatives to fossil-fuel technologies has revolutionized clean energy production using fuel cells. However, to date, the slow rate of the hydrogen oxidation reaction (HOR) in alkaline environments has hindered advances in alkaline fuel cell systems. Here, we address this by studying the trends in the activity of the HOR in alkaline environments. We demonstrate that it can be enhanced more than fivefold compared to state-of-the-art platinum catalysts. The maximum activity is found for materials (Ir and Pt0.1Ru0.9) with an optimal balance between the active sites that are required for the adsorption/dissociation of H2 and for the adsorption of hydroxyl species (OHad). We propose that the more oxophilic sites on Ir (defects) and PtRu material (Ru atoms) electrodes facilitate the adsorption of OHad species. Those then react with the hydrogen intermediates (Had) that are adsorbed on more noble surface sites.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Parsons, R. The rate of electrolytic hydrogen evolution and the heat of adsorption of hydrogen. Trans. Faraday Soc. 54, 1053–1063 (1958).
Gerischer, H. Mechanism of electrolytic discharge of hydrogen and adsorption energy of atomic hydrogen. Bull. Soc. Chim. Belg. 67, 506 (1958).
Conway, B. E. & Bockris, J. O. M. Electrolytic hydrogen evolution kinetics and its relation to the electronic and adsorptive properties of the metal. J. Chem. Phys. 26, 532–542 (1957).
Trasatti, S. Work function, electronegativity, and electrochemical behaviour of metals: III. Electrolytic hydrogen evolution in acid solutions. J. Electroanal. Chem. 39, 163–184 (1972).
Norskov, J. K., Bligaard, T., Rossmeisl, J. & Christensen, C. H. Towards the computational design of solid catalysts. Nature Chem. 1, 37–46 (2009).
Breiter, M. W. in Handbook of Fuel Cells (eds. Vielstich, W., Lamm, A. & Gasteiger, H.) 361–368 (Wiley, 2010).
Schmickler, W. & Santos, E. in Interfacial Electrochemistry 163–175 (Springer, 2010).
Wolfschmidt, H., Paschos, O. & Stimming, U. in Fuel Cell Science: Theory, Fundamentals, and Biocatalysis (eds Wieckowski, A. & Nørskov, J. K.) 1–70 (Wiley, 2010).
Conway, B. E. in Interfacial Electrochemistry: Theory, Experiment, and Applications (ed. Wieckowski, A.) 131–150 (CRC Press, 1999).
Savadogo, O. in Interfacial Electrochemistry: Theory, Experiment, and Applications (ed. Wieckowski, A.) 937–954 (CRC Press, 1999).
Subbaraman, R. et al. Enhancing hydrogen evolution activity in water splitting by tailoring Li+–Ni(OH)2–Pt interfaces. Science 334, 1256–1260 (2011).
Conway, B. E. & Tilak, B. V. Interfacial processes involving electrocatalytic evolution and oxidation of H2, and the role of chemisorbed H. Electrochim. Acta 47, 3571–3594 (2002).
Greeley, J. & Markovic, N. M. The road from animal electricity to green energy: combining experiment and theory in electrocatalysis. Energy Environ. Sci. 5, 9246–9256 (2012).
Petrii, O. A. & Tsirlina, G. A. Electrocatalytic activity prediction for hydrogen electrode reaction: intuition, art, science. Electrochim. Acta 39, 1739–1747 (1994).
Markovic, N. M., Sarraf, S. T., Gasteiger, H. A. & Ross, P. N. Hydrogen electrochemistry on platinum low-index single-crystal surfaces in alkaline solution. J. Chem. Soc. Faraday Trans. 92, 3719–3725 (1996).
Markovic, N. M., Grgur, B. N. & Ross, P. N. Temperature-dependent hydrogen electrochemistry on platinum low-index single-crystal surfaces in acid solutions. J. Phys. Chem. B 101, 5405–5413 (1997).
Sheng, W., Gasteiger, H. A. & Shao-Horn, Y. Hydrogen oxidation and evolution reaction kinetics on platinum: acid vs alkaline electrolytes. J. Electrochem. Soc. 157, B1529–B1536 (2010).
Barber, J. H. & Conway, B. E. Structural specificity of the kinetics of the hydrogen evolution reaction on the low-index surfaces of Pt single-crystal electrodes in 0.5 M dm−3 NaOH. J. Electroanal. Chem. 461, 80–89 (1999).
Schmidt, T. J., Ross, P. N. & Markovic, N. M. Temperature dependent surface electrochemistry on Pt single crystals in alkaline electrolytes: Part 2. The hydrogen evolution/oxidation reaction. J. Electroanal. Chem. 524, 252–260 (2002).
Subbaraman, R. et al. Trends in activity for the water electrolyser reactions on 3d M(Ni,Co,Fe,Mn) hydr(oxy)oxide catalysts. Nature Mater. 11, 550–557 (2012).
Danilovic, N. et al. Enhancing the alkaline hydrogen evolution reaction activity through the bifunctionality of Ni(OH)2/metal catalysts. Angew. Chem. Int. Ed. 124, 12663–12666 (2012).
Auinger, M. et al. Near-surface ion distribution and buffer effects during electrochemical reactions. Phys. Chem. Chem. Phys. 13, 16384–16394 (2011).
Angerstein-Kozlowska, H., Conway, B. E. & Hamelin, A. Electrocatalytic mediation of oxidation of H2 at gold by chemisorbed states of anions. J. Electroanal. Chem. 277, 233–252 (1990).
Marković, N. M. & Ross, P. N. Jr. Surface science studies of model fuel cell electrocatalysts. Surf. Sci. 45, 117–229 (2002).
Strmcnik, D. et al. The role of non-covalent interactions in electrocatalytic fuel-cell reactions on platinum. Nature Chem. 1, 466–472 (2009).
Wang, J. X., Marinković, N. S., Zajonz, H., Ocko, B. M. & Adžić, R. R. In situ X-ray reflectivity and voltammetry study of Ru(0001) surface oxidation in electrolyte solutions. J. Phys. Chem. B 105, 2809–2814 (2001).
Skúlason, E. et al. Modeling the electrochemical hydrogen oxidation and evolution reactions on the basis of density functional theory calculations. J. Phys. Chem. C 114, 18182–18197 (2010).
Marković, N. M. et al. Effect of temperature on surface processes at the Pt(111)−liquid interface: hydrogen adsorption, oxide formation, and CO oxidation. J. Phys. Chem. B 103, 8568–8577 (1999).
Marinkovic, N. S. et al. Hydrogen adsorption on single-crystal platinum electrodes in alkaline solutions. J. Electroanal. Chem. 330, 433–452 (1992).
Strmcnik, D. S. et al. Unique activity of platinum adislands in the CO electrooxidation reaction. J. Am. Chem. Soc. 130, 15332–15339 (2008).
Marković, N. M., Grgur, B. N., Lucas, C. A. & Ross, P. N. Electrooxidation of CO and H2/CO mixtures on Pt(111) in acid solutions. J. Phys. Chem. B 103, 487–495 (1999).
Strmcnik, D. S. et al. Unique activity of platinum adislands in the CO electrooxidation reaction. J. Am. Chem. Soc. 130, 15332–15339 (2008).
Arenz, M. et al. The effect of the particle size on the kinetics of CO electrooxidation on high surface area Pt catalysts. J. Am. Chem. Soc. 127, 6819–6829 (2005).
Climent, V., Attard, G. A. & Feliu, J. M. Potential of zero charge of platinum stepped surfaces: a combined approach of CO charge displacement and N2O reduction. J. Electroanal. Chem. 532, 67–74 (2002).
Gasteiger, H. A., Ross, P. N. & Cairns, E. J. LEIS and AES on sputtered and annealed polycrystalline Pt–Ru bulk alloys. Surf. Sci. 293, 67–80 (1993).
Subbaraman, R. et al. Origin of anomalous activities for electrocatalysts in alkaline electrolytes. J. Phys. Chem. C 116, 7, 22231–22237 (2012).
Acknowledgements
This work was supported by the Office of Science, Office of Basic Energy Sciences, Division of Materials Sciences, US Department of Energy (contract no. DE-AC02-06CH11357).
Author information
Authors and Affiliations
Contributions
D.S. and N.M.M. conceived and designed the experiments. D.S., M.U., D.v.D., N.D. and A.P.P. performed the experiments. C.W. contributed materials (Ir nanoparticles). D.S., R.S., V.R.S. and N.M.M. discussed the results and co-wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 2358 kb)
Rights and permissions
About this article
Cite this article
Strmcnik, D., Uchimura, M., Wang, C. et al. Improving the hydrogen oxidation reaction rate by promotion of hydroxyl adsorption. Nature Chem 5, 300–306 (2013). https://doi.org/10.1038/nchem.1574
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1574
This article is cited by
-
Pt single atoms coupled with Ru nanoclusters enable robust hydrogen oxidation for high-performance anion exchange membrane fuel cells
Nano Research (2024)
-
Promoting water dissociation for efficient solar driven CO2 electroreduction via improving hydroxyl adsorption
Nature Communications (2023)
-
Atomic metal–non-metal catalytic pair drives efficient hydrogen oxidation catalysis in fuel cells
Nature Catalysis (2023)
-
Understanding hydrogen electrocatalysis by probing the hydrogen-bond network of water at the electrified Pt–solution interface
Nature Energy (2023)
-
Embedding oxophilic rare-earth single atom in platinum nanoclusters for efficient hydrogen electro-oxidation
Nature Communications (2023)