Abstract
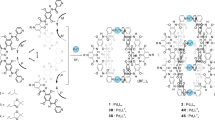
Long-range communication of stereochemical information is common in biological systems, particularly in enzyme-catalysed reactions. Here, we report the remote control of the dynamic chirality of metal centres, coordinated by 2,2′-bipyridine ligands bearing dynamic helical oligopeptides. The helical chirality of the oligopeptides is controlled by a stereocentre remote from the metal. We show that when a mixture of chiral and achiral peptide ligands is used, both the chirality of the metal centre and that of the achiral oligopeptide helices are significantly amplified. The amplification mechanism relies on several steps of chirality induction, first from a single chiral peptide to the helicity of an oligopeptide through the induction of propeller chirality at the metal centre, then on to induction of helical chirality in an achiral oligopeptide.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Rebek, J. Jr Simultaneous encapsulation: molecules held at close range. Angew. Chem. Int. Ed. 44, 2068–2078 (2005).
Caulder, D. L. & Raymond, K. N. Supermolecules by design. Acc. Chem. Res. 32, 975–982 (1999).
Fujita, M., Tominaga, M., Hori, A. & Therrien, B. Coordination assemblies from a Pd(II)-cornered square complex. Acc. Chem. Res. 38, 369–378 (2005).
Mateos-Timoneda, M. A., Crego-Calama, M. & Reinhoudt, D. N. Supramolecular chirality of self-assembled systems in solution. Chem. Soc. Rev. 33, 363–372 (2004).
Lehn, J. M. Supramolecular Chemistry: Concepts and Perspectives (VCH, 1995).
Albrecht, M. ‘Let's twist again’—double-stranded, triple-stranded, and circular helicates. Chem. Rev. 101, 3457–3497 (2001).
Palmans, A. R. A. & Meijer, E. W. Amplification of chirality in dynamic supramolecular aggregates. Angew. Chem. Int. Ed. 46, 8948–8968 (2007).
Pijper, D. & Feringa, B. L. Control of dynamic helicity at the macro- and supramolecular level. Soft Matter 4, 1349–1372 (2008).
Stang, P. J., Olenyuk, B., Muddiman, D. C. & Smith R. D. Transition-metal-mediated rational design and self-assembly of chiral, nanoscale supramolecular polyhedra with unique T symmetry. Organometallics 16, 3094–3096 (1997).
Werner, A. Zur kenntnis des asymmetrischen kobaltatoms. I. Chem. Ber. 44, 1887–1898 (1911).
Knof, U. & von Zelewsky, A. Predetermined chirality at metal center. Angew. Chem. Int. Ed. 38, 302–322 (1999).
Mizuno, T., Takeuchi, M., Hamachi, I., Nakashima, K. & Shinkai, S. Chiroselective transcription of the sugar structure to Δ- or Λ-[CoIII(bpy)3]3+ using a boronic acid–sugar template interaction. Chem. Commun. 1793–1794 (1997).
Telfer, S. G., Bernardinelli, G. & Williams, A. F. Diastereospecific synthesis of amino acid substituted 2,2′-bipyridyl complexes. Chem. Commun. 1498–1499 (2001).
Inai, Y. et al. Induction of one-handed helical screw sense in achiral peptide through the domino effect based on interacting its N-terminal amino group with chiral carboxylic acid. J. Am. Chem. Soc. 122, 11731–17732 (2000).
Inai, Y. et al. Noncovalent domino effect on helical screw sense of chiral peptides possessing C-terminal chiral residue. J. Am. Chem. Soc. 124, 2466–2473 (2002).
Ousaka, N., Inai, Y. & Kuroda, R. Chain-terminus triggered chiral memory in an optically inactive 310-helical peptide. J. Am. Chem. Soc. 130, 12266–12267 (2008).
Ousaka, N. & Inai, Y. Transfer of noncovalent chiral information along an optically inactive helical peptide chain: allosteric control of asymmetry of the C-terminal site by external molecule that binds to the N-terminal site. J. Org. Chem. 74, 1429–1439 (2009).
Inai, Y., Komori, H. & Ousaka, N. Control of helix sense in protein-mimicking backbone by the noncovalent chiral effect. Chem. Rec. 7, 191–202 (2007).
Mazaleyrat, J.-P. et al. Induced axial chirality in the biphenyl core of the Ca-tetrasubstituted α-amino acid residue Bip and subsequent propagation of chirality in (Bip)n/Val oligopeptides. J. Am. Chem. Soc. 126, 12874–12879 (2004).
Clayden, J., Lund, A., Vallverdú, L. & Helliwell, M. Ultra-remote stereocontrol by conformational communication of information along a carbon chain. Nature 431, 966–971 (2004).
Clayden, J., Castellanos, A., Solà, J. & Morris, G. A. Quantifying end-to-end conformational communication of chirality through an achiral peptide chain. Angew. Chem. Int. Ed. 48, 5962–5965 (2009).
Solà, J., Helliwell, M. & Clayden, J. N- versus C-terminal control over the screw-sense preference of the configurationally achiral, conformationally helical peptide motif Aib8GlyAib8 . J. Am. Chem. Soc. 132, 4548–4549 (2010).
Clayden, J. Transmission of stereochemical information over nanometre distances in chemical reactions. Chem. Soc. Rev. 38, 817–829 (2009).
Okamoto, Y., Matsuda, M., Nakano, T. & Yashima, E. Asymmetric polymerization of isocyanates with optically active anionic initiators. Polym. J. 25, 391–396 (1993).
Pijper, D. & Feringa, B. L. Molecular transmission: controlling the twist sense of a helical polymer with a single light-driven molecular motor. Angew. Chem. Int. Ed. 46, 3693–3696 (2007).
Dolain, C., Jiang, H., Leger, J.-M., Guionneau, P. & Huc, I. Chiral induction in quinoline-derived oligoamide foldamers: assignment of helical handedness and role of steric effects. J. Am. Chem. Soc. 127, 12943–12951 (2005).
Kamikawa, K. et al. Induction of one-handed helical oligo(p-benzamide)s by domino effect based on planar-axial-helical chirality relay. Chem. Commun. 1201–1203 (2009).
Karle, I. L. & Balaram, P. Structural characteristics of α-helical peptide molecules containing Aib residues. Biochemistry 29, 6747–6756 (1990).
Toniolo, C. & Benedetti, E. The polypeptide 310-helix. Trends Biochem. Sci. 16, 350–353 (1991).
Paul, P. K. C. et al. Stereochemically constrained peptides. Theoretical and experimental studies on the conformations of peptides containing 1-aminocyclohexanecarboxylic acid. J. Am. Chem. Soc. 108, 6363–6370 (1986).
Pengo, B. et al. Linear oligopeptides. Part 406. Helical screw sense of peptide molecules: the pentapeptide system (Aib)4/L-Val[L-(αMe)Val] in solution. J. Chem. Soc. Perkin Trans. 2, 1651–1657 (1998).
Hirschberg, J. H. K. K. et al. Helical self-assembled polymers from cooperative stacking of hydrogen-bonded pairs. Nature 407, 167–170 (2000).
Green, M. M. et al. A helical polymer with a cooperative response to chiral information. Science 268, 1860–1866 (1995).
Yashima, E., Maeda, K., Iida, H., Furusho, Y. & Nagai, K. Helical polymers: synthesis, structures, and functions. Chem. Rev. 109, 6102–6211 (2009).
Cornelissen, J. J. L. M., Rowan, A. E., Nolte, R. J. M. & Sommerdijk, N. A. J. M. Chiral architectures from macromolecular building blocks. Chem. Rev. 101, 4039–4070 (2001).
Fujiki, M. Optically active polysilylenes: state-of-the-art chiroptical polymers. Macromol. Rapid Commun. 22, 539–563 (2001).
Mateos-Timoneda, M. A., Crego-Calama, M. & Reinhoudt, D. N. Controlling the amplification of chirality in hydrogen-bonded assemblies. Supramol. Chem. 17, 67–79 (2005).
Rudick, J. G. & Percec, V. Helical chirality in dendronized polyarylacetylenes. New J. Chem. 31, 1083–1096 (2007).
Ziegler, M. & von Zelewsky, A. Charge-transfer excited state properties of chiral transition metal coordination compounds studied by chiroptical spectroscopy. Coord. Chem. Rev. 177, 257–300 (1998).
Ahn, D.-R., Kim, T. W. & Hong, J.-I. Induction of diastereoselectivity in Fe(II) tris(amino acid-bipyridine) complexes. J. Org. Chem. 66, 5008–5011 (2001).
Constable, E. C., Frantz, R., Housecroft, C. E., Lacour, J. & Mahmood, A. Chiral induction in a ribose-decorated metallostar through intrinsic and interionic diastereomeric interactions. Inorg. Chem. 43, 4817–4819 (2004).
Drahoňovský, D. et al. Stereoselectivity in the formation of tris-diimine complexes of Fe(II), Ru(II), and Os(II) with a C2-symmetric chiral derivative of 2,2′-bipyridine. Dalton Trans. 1444–1454 (2006).
Kopple, K. D. & Schamper, T. J. Proton magnetic resonance line broadening produced by association with a nitroxide radical in studies of amide and peptide conformation. J. Am. Chem. Soc. 94, 3644–3646 (1972).
Toniolo, C., Formaggio, F., Crisma, M., Schoemaker, H. S. & Kamphuis, J. The p-bromobenzamido chromophore as a circular dichroic probe for the assignment of the screw sense of helical peptides. Tetrahedron Asymm. 5, 507–510 (1994).
Wüthrich, K. NMR of Proteins and Nucleic Acids (John Wiley & Sons, 1986).
Uppadine, L. H., Drew, M. G. B. & Beer, P. D. Anion selective properties of ruthenium(II) tris(5,5′-diamide-2,2′-bipyridine) receptors dictated by solvent and amide substituent. Chem. Commun. 291–292 (2001).
Inai, Y., Ousaka, N. & Okabe, T. Mechanism for the noncovalent domino effect: new paradigm for the chiral role of the N-terminal segment in a 310-helix. J. Am. Chem. Soc. 125, 8151–8162 (2003).
Jaun, B. et al. Studies on the conformation of Boc-protected (S)-(+)-isovaline homopeptide methyl esters in the solid state and in solution. Liebigs Ann. Recueil 1697–1710 (1997).
Acknowledgements
This work was supported in part by a Grant-in-Aid for Scientific Research (S) from the Japan Society for the Promotion of Science (JSPS) (no. 20225006) and the Global COE Program ‘Elucidation and Design of Materials and Molecular Functions’ of the Ministry of Education, Culture, Sports, Science, and Technology, Japan. N.O. expresses thanks for a JSPS Postdoctoral Fellowship for Young Scientists (no. 2692). The authors thank K. Yoza and Y. Furusho for their help with X-ray crystallographic analysis.
Author information
Authors and Affiliations
Contributions
E.Y. designed and directed the project. N.O. conceived and designed the experiments. N.O., Y.T. and H.I. performed the experiments. E.Y. and N.O. analysed the data and co-wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 6459 kb)
Supplementary information
Crystallographic data for Fe(Ligand3)3Cl2 (CIF 154 kb)
Rights and permissions
About this article
Cite this article
Ousaka, N., Takeyama, Y., Iida, H. et al. Chiral information harvesting in dendritic metallopeptides. Nature Chem 3, 856–861 (2011). https://doi.org/10.1038/nchem.1146
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1146
This article is cited by
-
Stapling strategy for slowing helicity interconversion of α-helical peptides and isolating chiral auxiliary-free one-handed forms
Nature Communications (2023)
-
Hierarchical communication of chirality for aromatic oligoamide sequences
Nature Communications (2021)
-
Induction and modulation of supramolecular chirality in side-chain azobenzene polymers through the covalent chiral domino effect
Science China Chemistry (2021)
-
Phosphoramidite-based photoresponsive ligands displaying multifold transfer of chirality in dynamic enantioselective metal catalysis
Nature Catalysis (2020)
-
Ligand-modulated conformational switching in a fully synthetic membrane-bound receptor
Nature Chemistry (2017)