Abstract
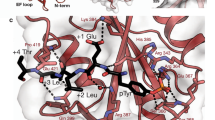
SAP (SLAM-associated protein) is a small lymphocyte-specific signalling molecule that is defective or absent in patients with X-linked lymphoproliferative syndrome (XLP)1,2,3. Consistent with its single src homology 2 (SH2) domain architecture and unusually high affinity for SLAM (also called CD150), SAP has been suggested to function by blocking binding of SHP-2 or other SH2-containing signalling proteins to SLAM receptors1,4. Additionally, SAP has recently been shown to be required for recruitment and activation of the Src-family kinase FynT after SLAM ligation5. This signalling 'adaptor' function has been difficult to conceptualize, because unlike typical SH2-adaptor proteins, SAP contains only a single SH2 domain and lacks other recognized protein interaction domains or motifs. Here, we show that the SAP SH2 domain binds to the SH3 domain of FynT and directly couples FynT to SLAM. The crystal structure of a ternary SLAM–SAP–Fyn-SH3 complex reveals that SAP binds the FynT SH3 domain through a surface–surface interaction that does not involve canonical SH3 or SH2 binding interactions. The observed mode of binding to the Fyn-SH3 domain is expected to preclude the auto-inhibited conformation of Fyn, thereby promoting activation of the kinase after recruitment. These findings broaden our understanding of the functional repertoire of SH3 and SH2 domains.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
Sayos, J. et al. The X-linked lymphoproliferative-disease gene product SAP regulates signals induced through the co-receptor SLAM. Nature 395, 462–469 (1998).
Coffey, A.J. et al. Host response to EBV infection in X-linked lymphoproliferative disease results from mutations in an SH2-domain encoding gene. Nature Genet. 20, 129–135 (1998).
Nichols, K.E. et al. Inactivating mutations in an SH2 domain-encoding gene in X-linked lymphoproliferative syndrome. Proc. Natl Acad. Sci. USA 95, 13765–13770 (1998).
Poy, F. et al. Crystal structures of the XLP protein SAP reveal a class of SH2 domains with extended, phosphotyrosine-independent sequence recognition. Mol. Cell 4, 555–561 (1999).
Latour, S. et al. Regulation of SLAM-mediated signal transduction by SAP, the X-linked lymphoproliferative gene product. Nature Immunol. 2, 681–690 (2001).
Morra, M. et al. X-linked lymphoproliferative disease: a progressive immunodeficiency. Annu. Rev. Immunol. 19, 657–682 (2001).
Sidorenko, S.P. & Clark, E.A. Characterization of a cell surface glycoprotein IPO-3, expressed on activated human B and T lymphocytes. J. Immunol. 151, 4614–4624 (1993).
Wang, N. et al. CD150 is a member of a family of genes that encode glycoproteins on the surface of hematopoietic cells. Immunogenetics 53, 382–394 (2001).
Cocks, B.G. et al. A novel receptor involved in T-cell activation. Nature 376, 260–263 (1995).
Punnonen, J. et al. Soluble and membrane-bound forms of signaling lymphocytic activation molecule (SLAM) induce proliferation and Ig synthesis by activated human B lymphocytes. J. Exp. Med. 185, 993–1004 (1997).
Mavaddat, N. et al. Signaling lymphocytic activation molecule (CDw150) is homophilic but self-associates with very low affinity. J. Biol. Chem. 275, 28100–28109 (2000).
Tatsuo, H., Ono, N., Tanaka, K. & Yanagi, Y. SLAM (CDw150) is a cellular receptor for measles virus. Nature 406, 893–897 (2000).
Wu, C. et al. SAP controls T cell responses to virus and terminal differentiation of TH2 cells. Nature Immunol. 2, 410–414 (2001).
Czar, M.J. et al. Altered lymphocyte responses and cytokine production in mice deficient in the X-linked lymphoproliferative disease gene SH2D1A/DSHP/SAP. Proc. Natl Acad. Sci. USA 98, 7449–7454 (2001).
Sayos, J. et al. Potential pathways for regulation of NK and T cell responses: differential X-linked lymphoproliferative syndrome gene product SAP interactions with SLAM and 2B4. Int. Immunol. 12, 1749–1757 (2000).
Sayos, J. et al. Cell surface receptors Ly-9 and CD84 recruit the X-linked lymphoproliferative disease gene product SAP. Blood 97, 3867–3874 (2001).
Morra, M. et al. Structural basis for the interaction of the free SH2 domain EAT-2 with SLAM receptors in hematopoietic cells. EMBO J. 20, 5840–5852 (2001).
Morra, M. et al. Characterization of SH2D1A missense mutations identified in X-linked lymphoproliferative disease patients. J. Biol. Chem. 276, 36809–36816 (2001).
Noble, M., E. M., Musacchio, A., Saraster, M., Courtneidge, S.A. & Wierenga, R.K. Crystal structure of the SH3 domain in human Fyn; comparison of the three-dimensional structure of SH3 domains in tyrosine kinases and spectrin. EMBO J. 12, 2617–2624 (1993).
Moarefi, I. et al. Activation of the Src-family tyrosine kinase Hck by SH3 domain displacement. Nature 385, 650–653 (1997).
Holdorf, A.D., Lee, K.H., Burack, W.R., Allen, P.M. & Shaw, A.S. Regulation of Lck activity by CD4 and CD28 in the immunological synapse. Nature Immunol. 3, 259–264 (2002).
Xu, W., Harrison, S.C. & Eck, M.J. Three-dimensional structure of the tyrosine kinase c-Src. Nature 385, 595–602 (1997).
Hof, P., Pluskey, S., Dhe-Paganon, S., Eck, M.J. & Shoelson, S.E. Crystal structure of the tyrosine phosphatase SHP-2. Cell 92, 441–450 (1998).
Howie, D. et al. Antibodies directed at murine CD150(SLAM) mediate cell co-stimulation in both a SAP-dependent and SAP-independent fashion. Blood (in the press).
Howie, D. et al. Molecular dissection of the signaling and costimulatory functions of CD150 (SLAM): CD150/SAP binding and CD150-mediated costimulation. Blood 99, 957–965 (2002).
Nagy, N. et al. SH2D1A and SLAM protein expression in human lymphocytes and derived cell lines. Int. J. Cancer 88, 439–447 (2000).
Navaza, J. Implementation of molecular replacement in AMoRe. Acta Crystallogr. D Biol. Crystallogr. 57, 1367–1372 (2001).
Jones, T.A. & Kjeldgaard, M. Electron-density map interpretation. Methods Enzymol. 277, 173–208 (1997).
Brunger, A.T. et al. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr. D. Biol. Crystallogr. 54, 905–921 (1998).
Nagy, N. et al. The X-linked lymphoproliferative disease gene product SAP is expressed in activated T and NK cells. Immunol. Lett. 82, 141–147 (2002).
Acknowledgements
We thank S.J. Freedman for assistance with the ITC experiments and helpful discussions. This work was supported in part by grants from the National Institutes of Health (M.J.E. and C.T.) and the Hungarian Academy of Sciences (A.L.). M.J.E. is a recipient of the Leukaemia and Lymphoma Society Scholar Award. Correspondence and requests for materials should be addressed to M.J.E.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Chan, B., Lanyi, A., Song, H. et al. SAP couples Fyn to SLAM immune receptors. Nat Cell Biol 5, 155–160 (2003). https://doi.org/10.1038/ncb920
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb920
This article is cited by
-
SH3 domain regulation of RhoGAP activity: Crosstalk between p120RasGAP and DLC1 RhoGAP
Nature Communications (2022)
-
Role of NKT cells in cancer immunotherapy—from bench to bed
Medical Oncology (2022)
-
Src Family Protein Kinase Controls the Fate of B Cells in Autoimmune Diseases
Inflammation (2021)
-
NK cell recognition of hematopoietic cells by SLAM-SAP families
Cellular & Molecular Immunology (2019)
-
Structural basis of myelin-associated glycoprotein adhesion and signalling
Nature Communications (2016)