Abstract
Membrane proteins that are degraded in the vacuole of Saccharomyces cerevisiae are sorted into discrete intralumenal vesicles, analogous to the internal membranes of multi-vesiculated bodies (MVBs). Recently, it has shown that the attachment of ubiquitin (Ub) mediates sorting into lumenal membranes1. We describe a complex of Vps27p and Hse1p that localizes to endosomal compartments and is required for the recycling of Golgi proteins, formation of lumenal membranes and sorting of ubiquitinated proteins into those membranes. The Vps27p–Hse1p complex binds to Ub and requires multiple Ub Interaction Motifs (UIMs). Mutation of these motifs results in specific defects in the sorting of ubiquitinated proteins into the vacuolar lumen. However, the recycling of Golgi proteins and the generation of lumenal membranes proceeds normally in Δ UIM mutants. These data support a model in which the Vps27p–Hse1p complex has multiple functions at the endosome, one of which is as a sorting receptor for ubiquitinated membrane proteins destined for degradation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hicke, L. Cell 106, 527–530 (2001).
Piper, R. C. & Luzio, J. P. Traffic 2, 612–621 (2001).
Asao, H. et al. J. Biol. Chem. 272, 32785–32791 (1997).
Takata, H., Kato, M., Denda, K. & Kitamura, N. Genes Cells 5, 57–69 (2000).
Lohi, O. et al. J. Biol. Chem. 273, 21408–21415 (1998).
Piper, R. C., Cooper, A. A., Yang, H. & Stevens, T. H. J. Cell Biol. 131, 603–617 (1995).
Cooper, A. A. & Stevens, T. H. J. Cell Biol. 133, 529–541 (1996).
Chen, L. & Davis, N. G. Traffic 3, 110–123 (2002).
Komada, M. & Kitamura, N. Biochem. Biophys. Res. Commun. 281, 1065–1069 (2001).
Uetz, P. et al. Nature 403, 623–627 (2000).
Dell'Angelica, E. C. & Payne, G. S. Cell 106, 395–398 (2001).
Hofmann, K. & Falquet, L. Trends Biochem. Sci. 26, 347–350 (2001).
Lloyd, T. E. et al. J. Cell 108, 261–269 (2002).
Polo, S. et al. Nature 416, 451–455 (2002).
Shih, S. C. et al. Nature Cell Biol. 4, 389–393 (2002).
Raiborg, C. et al. Nature Cell Biol. 4,394–398 (2002).
Urbanowski, J. L. & Piper, R. C. J. Biol. Chem. 274, 38061–38070 (1999).
Urbanowski, J. L. & Piper, R. C. Traffic 2, 622–630 (2001).
Simeon, A., van der Klei, I. J., Veenhuis, M. & Wolf, D. H. FEBS Lett. 301, 231–235 (1992).
Grant, A. M., Hanson, P. K., Malone, L. & Nichols, J. W. Traffic 2, 37–50 (2001).
Kean, L. S. et al. J. Cell Biol. 138, 255–270 (1997).
Reggiori, F. & Pelham, H. R. EMBO J. 20, 5176–5186 (2001).
Katzmann, D. J., Babst, M. & Emr, S. D. Cell 106, 145–155 (2001).
Stevens, T. H., Rothman, J. H., Payne, G. S. & Schekman, R. J. Cell Biol. 102, 1551–1557 (1986).
Raymond, C. K., Howald-Stevenson, I., Vater, C. A. & Stevens, T. H. Mol. Biol. Cell 3, 1389–1402 (1992).
Sikorski, R. S. & Hieter, P. Genetics 122, 19–27 (1989).
Mullock, B. M. et al. Mol. Cell. Biol. 11, 3137–3153 (2000).
Smith, D. B. & Johnson, K. S. Gene 67, 31–40 (1988).
Wang, J. & Wilkinson, M. F. Biotechniques 29, 976–978 (2000).
Piper, R. C., Bryant, N. J. & Stevens, T. H. J. Cell Biol. 138, 531–545 (1997).
Acknowledgements
We thank S. Moye-Rowley, B. Cohen, T. Stevens and L. Weisman for helpful suggestions. We also thank the University of Iowa Central Microscopy Facility for technical assistance with electron microscopy. This work was supported by National Institutes of Health grant RO1 GM58202.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary figures
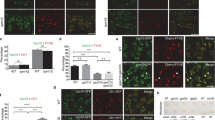
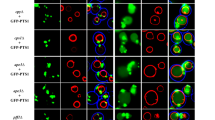
Figure 1. Hse1-GFP function and Immunolocalization of Vps27 in hse1 mutant cells. a, The (PDF 333 kb)
Figure 2. The Ub-independent sorting of Sna3-GFP is normal in ΔUIM cells.
Rights and permissions
About this article
Cite this article
Bilodeau, P., Urbanowski, J., Winistorfer, S. et al. The Vps27p–Hse1p complex binds ubiquitin and mediates endosomal protein sorting. Nat Cell Biol 4, 534–539 (2002). https://doi.org/10.1038/ncb815
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb815
This article is cited by
-
Identification of small compounds regulating the secretion of extracellular vesicles via a TIM4-affinity ELISA
Scientific Reports (2021)
-
Rab5-mediated endosome formation is regulated at the trans-Golgi network
Communications Biology (2019)
-
ESCRT-III accessory proteins regulate fungal development and plant infection in Fusarium graminearum
Current Genetics (2019)
-
Ist1 regulates ESCRT-III assembly and function during multivesicular endosome biogenesis in Caenorhabditis elegans embryos
Nature Communications (2017)
-
Bifurcation of the endocytic pathway into Rab5-dependent and -independent transport to the vacuole
Nature Communications (2014)