Abstract
Brown fat dissipates energy as heat and protects against obesity. Here, we identified nuclear factor I-A (NFIA) as a transcriptional regulator of brown fat by a genome-wide open chromatin analysis of murine brown and white fat followed by motif analysis of brown-fat-specific open chromatin regions. NFIA and the master transcriptional regulator of adipogenesis, PPARγ, co-localize at the brown-fat-specific enhancers. Moreover, the binding of NFIA precedes and facilitates the binding of PPARγ, leading to increased chromatin accessibility and active transcription. Introduction of NFIA into myoblasts results in brown adipocyte differentiation. Conversely, the brown fat of NFIA-knockout mice displays impaired expression of the brown-fat-specific genes and reciprocal elevation of muscle genes. Finally, expression of NFIA and the brown-fat-specific genes is positively correlated in human brown fat. These results indicate that NFIA activates the cell-type-specific enhancers and facilitates the binding of PPARγ to control the brown fat gene program.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Cannon, B. & Nedergaard, J. Brown adipose tissue: function and physiological significance. Physiol. Rev. 84, 277–359 (2004).
Nedergaard, J., Bengtsson, T. & Cannon, B. Unexpected evidence for active brown adipose tissue in adult humans. Am. J. Physiol. Endocrinol. Metab. 293, E444–E452 (2007).
van Marken Lichtenbelt, W. D. et al. Cold-activated brown adipose tissue in healthy men. N. Engl. J. Med. 360, 1500–1508 (2009).
Cypess, A. M. et al. Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 360, 1509–1517 (2009).
Virtanen, K. A. et al. Functional brown adipose tissue in healthy adults. N. Engl. J. Med. 360, 1518–1525 (2009).
Saito, M. et al. High incidence of metabolically active brown adipose effects of cold exposure and adiposity. Diabetes 58, 1526–1531 (2009).
van der Lans, A. A. J. J. et al. Cold acclimation recruits human brown fat and increases nonshivering thermogenesis. J. Clin. Invest. 123, 3395–3403 (2013).
Hanssen, M. J. W. et al. Short-term cold acclimation improves insulin sensitivity in patients with type 2 diabetes mellitus. Nat. Med. 21, 6–10 (2015).
Cypess, A. M. et al. Activation of human brown adipose tissue by a β3-adrenergic receptor agonist. Cell Metab. 21, 33–38 (2015).
Seale, P. et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature 454, 961–967 (2008).
Seale, P. et al. Transcriptional control of brown fat determination by PRDM16. Cell Metab. 6, 38–54 (2007).
Harms, M. J. et al. PRDM16 binds MED1 and controls chromatin architecture to determine a brown fat transcriptional program. Genes Dev. 29, 298–307 (2015).
Ohno, H., Shinoda, K., Spiegelman, B. M. & Kajimura, S. PPARγ agonists induce a white-to-brown fat conversion through stabilization of PRDM16 protein. Cell Metab. 15, 395–404 (2012).
Siersbæk, M. S. et al. Genome-wide profiling of peroxisome proliferator-activated receptor γ in primary epididymal, inguinal, and brown adipocytes reveals depot-selective binding correlated with gene expression. Mol. Cell. Biol. 32, 3452–3463 (2012).
Rajakumari, S. et al. EBF2 determines and maintains brown adipocyte identity. Cell Metab. 17, 562–574 (2013).
Giresi, P. G., Kim, J., McDaniell, R. M., Iyer, V. R. & Lieb, J. D. FAIRE (Formaldehyde-Assisted Isolation of Regulatory Elements) isolates active regulatory elements from human chromatin. Genome Res. 17, 877–885 (2007).
Villanueva, C. J. et al. Adipose subtype-selective recruitment of TLE3 or Prdm16 by PPARγ specifies lipid storage versus thermogenic gene programs. Cell Metab. 17, 423–435 (2013).
Kajimura, S. et al. Initiation of myoblast to brown fat switch by a PRDM16-C/EBP-β transcriptional complex. Nature 460, 1154–1158 (2009).
Gronostajski, R. M. Roles of the NFI/CTF gene family in transcription and development. Development 249, 31–45 (2000).
Buenrostro, J. D., Giresi, P. G., Zaba, L. C., Chang, H. Y. & Greenleaf, W. J. Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat. Methods 10, 1213–1218 (2013).
Heinz, S. et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell 38, 576–589 (2010).
Mullen, A. C. et al. Master transcription factors determine cell-type-specific responses to TGF-β signaling. Cell 147, 565–576 (2011).
das Neves, L. et al. Disruption of the murine nuclear factor I-A gene (Nfia) results in perinatal lethality, hydrocephalus, and agenesis of the corpus callosum. Proc. Natl Acad. Sci. USA 96, 11946–11951 (1999).
Harms, M. & Seale, P. Brown and beige fat: development, function and therapeutic potential. Nat. Med. 19, 1252–1263 (2013).
Nagano, G. et al. Activation of classical brown adipocytes in the adult human perirenal depot is highly correlated with PRDM16-EHMT1 complex expression. PLoS ONE 10, 1–13 (2015).
Jespersen, N. Z. et al. A classical brown adipose tissue mRNA signature partly overlaps with brite in the supraclavicular region of adult humans. Cell Metab. 17, 798–805 (2013).
Cypess, A. M. et al. Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nat. Med. 19, 635–639 (2013).
Tontonoz, P., Hu, E. & Spiegelman, B. M. Stimulation of adipogenesis in fibroblasts by PPAR γ2, a lipid-activated transcription factor. Cell 79, 1147–1156 (1994).
Liu, R. et al. Regulation of CSF1 promoter by the SWI/SNF-like BAF complex. Cell 106, 309–318 (2001).
Zaret, K. S. & Carroll, J. S. Pioneer transcription factors: establishing competence for gene expression. Genes Dev. 25, 2227–2241 (2011).
Soccio, R. E. et al. Genetic variation determines PPARγ function and anti-diabetic drug response in vivo. Cell 162, 33–44 (2015).
Maurano, M. T. et al. Large-scale identification of sequence variants influencing human transcription factor occupancy in vivo. Nat. Genet. 47, 1393–1401 (2015).
Grossman, S. R. et al. Systematic dissection of genomic features determining transcription factor binding and enhancer function. Proc. Natl Acad. Sci. USA 114, E1291–E1300 (2017).
Deplancke, B., Alpern, D. & Gardeux, V. The genetics of transcription factor DNA binding variation. Cell 166, 538–554 (2016).
Harms, M. J. et al. Prdm16 is required for the maintenance of brown adipocyte identity and function in adult mice. Cell Metab. 19, 593–604 (2014).
Wang, W. et al. Ebf2 is a selective marker of brown and beige adipogenic precursor cells. Proc. Natl Acad. Sci. USA 111, 14466–14471 (2014).
Sunadome, K., Suzuki, T., Usui, M., Ashida, Y. & Nishida, E. Antagonism between the master regulators of differentiation ensures the discreteness and robustness of cell fates. Mol. Cell 54, 526–535 (2014).
Graves, R. A., Tontonoz, P., Ross, S. R. & Spiegelman, B. M. Identification of a potent adipocyte-specific enhancer: involvement of an NF-1-like factor. Genes Dev. 5, 428–437 (1991).
Waki, H. et al. Global mapping of cell type-specific open chromatin by FAIRE-seq reveals the regulatory role of the NFI family in adipocyte differentiation. PLoS Genet. 7, e1002311 (2011).
Pradhan, R. N. et al. Dissecting the brown adipogenic regulatory network using integrative genomics. Sci. Rep. 7, 42130 (2017).
Zhou, J. et al. Nuclear factor I-C reciprocally regulates adipocyte and osteoblast differentiation via control of canonical Wnt signaling. FASEB J. 31, 1939–1952 (2017).
Wahl, S. et al. Epigenome-wide association study of body mass index, and the adverse outcomes of adiposity. Nature 541, 81–86 (2017).
Tseng, Y. H., Ueki, K., Kriauciunas, K. M. & Kahn, C. R. Differential roles of insulin receptor substrates in the anti-apoptotic function of insulin-like growth factor-1 and insulin. J. Biol. Chem. 277, 31601–31611 (2002).
Ohno, H., Shinoda, K., Ohyama, K., Sharp, L. Z. & Kajimura, S. EHMT1 controls brown adipose cell fate and thermogenesis through the PRDM16 complex. Nature 504, 163–167 (2013).
Zhang, Y. et al. Model-based analysis of ChIP-Seq (MACS). Genome Biol. 9, R137 (2008).
Liu, T. et al. Cistrome: an integrative platform for transcriptional regulation studies. Genome Biol. 12, R83 (2011).
Bailey, T. L. & MacHanick, P. Inferring direct DNA binding from ChIP-seq. Nucleic Acids Res. 40, 1–10 (2012).
Wingender, E. et al. TRANSFAC: an integrated system for gene expression regulation. Nucleic Acids Res. 28, 316–319 (2000).
Huang, D. W., Lempicki, R. A. & Sherman, B. T. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4, 44–57 (2009).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Reich, M. et al. GenePattern 2.0. Nat. Genet. 38, 500–501 (2006).
Acknowledgements
We are grateful to S. Mandrup for her suggestion about our study. We thank K. Shiina and K. Tatsuno for their help in library preparation for high-throughput sequencing, S. Fukuda and T. Umehara for their help in computational analysis, K. Ueki, S. Kajimura and C. Villanueva for providing cells and plasmids, K. Nakashima for his suggestion regarding NFIA-KO mice, and T. Sugiyama, T. Kubota and N. Kubota for their help in animal experiments. We also thank T. Wada for his technical assistance. This work is funded by an AMED-CREST research grant from the Japan Agency for Medical Research and Development (AMED) to T.Y.; by a grant-in-aid for scientific research (B) from the Japan Society for the Promotion of Science (JSPS), grant number 25293209 to H.W.; by a grant-in-aid for JSPS fellows from JSPS, grant number 15J02835 to Y.Hiraike; and by a junior scientist development grant from the Japan Diabetes Society to Y.Hiraike. Y.Hiraike has been supported by a research fellowship from JSPS. The Centre of Inflammation and Metabolism (CIM) and the Centre for Physical Activity Research (CFAS), Department of Infectious Diseases, Rigshospitalet is supported by a grant from TrygFonden. CIM/CFAS is a member of DD2—the Danish Center for Strategic Research in Type 2 Diabetes (the Danish Council for Strategic Research, grant no. 09-067009 and 09-075724). T.J.L. has been supported by a research grant from the Danish Diabetes Academy supported by the Novo Nordisk Foundation. The Novo Nordisk Foundation Center for Basic Metabolic Research is supported by an unconditional grant from the Novo Nordisk Foundation to University of Copenhagen.
Author information
Authors and Affiliations
Contributions
Y.Hiraike and H.W. designed the study. Y.Hiraike., H.W., J.Y., K.M., H.K., W.S., Y.Hirota and T.A. performed experiments. M.N., R.N., K.S., S.Y., S.T., Y.Hiraike and H.W. performed computational analysis. G.N., H.O., K.O. and M.Y. performed analysis of human perirenal BAT. A.P.W., Y.-H.T. and A.M.C. performed analysis of human neck BAT and WAT. T.J.L., N.Z.J. and C.S. performed analysis of human brown and white adipocytes. Y.Hiraike and H.W. wrote the manuscript. H.A., T.Y. and T.K. supervised all aspects of this work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 GO analysis of the in vivo FAIRE peaks, and changes in expression levels of Nfia after challenge.
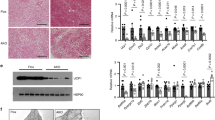
(a) Top GO terms of genes near BAT-specific FAIRE peaks and iWAT- or eWAT- specific FAIRE peaks. (b) Top GO terms of genes near BAT FAIRE peaks and iWAT or eWAT FAIRE peaks. The genome-wide analyses were performed once based on the FAIRE-seq dataset. (c) RT-qPCR of Nfia and Ucp1 before and after mice are challenged at 4 °C for 4 hours. (mean ± S.E.M.; N = 5 mice for room temperature and 6 mice for cold challenge, respectively; ∗ p < 0.05, ∗∗ p < 0.01). The representative results of two independent experiments are shown. (d) RT-qPCR analysis of Nfia and Ucp1 of indicated tissues in mice treated with saline or β3 agonist CL316,243, 1 mg/kg body weight, intraperitoneal injection, for 7 days. (mean ± S.E.M.; N = 5 mice per group; ∗ p < 0.05, ∗∗ p < 0.01). The representative results of two independent experiments are shown. (e) Western blot analysis of NFIA and UCP1. β-actin was used as a loading control. The representative images of two independent experiments are shown.
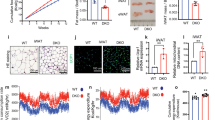
Supplementary Figure 2 Gain- and loss-of-function experiments showed the effect of NFIA on the brown fat gene program.
(a) Hierarchal clustering analysis of genes up- or down-regulated by NFIA. For example, genes up-regulated NFIA (Cluster A) include Ucp1, Ppargc1a, and Adrb3—the vital components for the induction of thermogenesis in response to adrenergic stimulus, as well as Pparg—the master regulator of adipogenesis. In contrast, Cluster B represents genes down-regulated by NFIA, including critical myogenic genes such as Myog, Myh1, and Myl1. Note that the clusters of genes up-regulated by NFIA largely overlap with BAT-selective genes (p = 9.9 × 10−28), and the cluster of genes down-regulated by NFIA overlap with SKM-selective genes (p = 2.3 × 10−32). The definition of BAT- and SKM- selective genes (N = 254 and N = 312, respectively) are shown in the methods. The analyses were performed once based on the RNA-seq dataset. (b) Top GO terms of genes up- or down-regulated by introduction of NFIA into C2C12 myoblasts. The analyses were performed once based on the RNA-seq dataset. (c) Control and NFIA-expressing 3T3-F442A adipocytes were stained with Oil Red O seven days after inducing adipocyte differentiation. Scale bar, 50 μm. (d–f) Nfia (d), general adipocyte genes (e) and the brown-fat-specific genes (f) were quantified by RT-qPCR at the indicated time course (mean ± S.E.M.; N = 3 independent samples; ∗ p < 0.05, ∗∗ p < 0.01). (g) Immortalized, differentiated brown adipocytes were electroporated with a control siRNA or a siRNA for NFIA and stained with Oil Red O. Scale bar, 50 μm. (h–j) Nfia (h), Pparg (i), Cidea and Ucp1 (j) were quantified by RT-qPCR at the indicated time course (mean ± S.E.M.; N = 3 independent samples; ∗ p < 0.05, ∗∗ p < 0.01).
Supplementary Figure 3 ChIP-seq analysis of NFI, PPARγ and other transcription factors.
(a) Genomic location of PPARγ binding sites in brown adipocytes at day 0 and day 6 of differentiation. (b) Enriched known motifs within NFIA binding sites in NFIA-expressing myoblasts at day 7. (c) Enriched de novo motifs within NFIA binding sites in NFIA-expressing myoblasts at day 7. (d) Enriched known motifs within PPARγ binding sites in brown adipocytes at day 6. (e) Venn diagram showing the overlap of NFI binding sites in brown adipocytes and NFIA binding sites in NFIA-expressing C2C12 myoblasts within BAT FAIRE peaks. (f–j) Venn diagram showing the overlap of indicated transcription factors at day0 and day 6 of differentiation. (k) Venn diagram showing the overlap of NFI and PPARγ ChIP-seq peaks in 3T3-F442A white adipocytes at day 6. (l) Bar graph showing the number of co-localizing sites per gene within ±50 kb of BAT- and WAT-selective genes stratified by the fold changes of gene expression. (m) To the left, NF-1 motif density around the NFI binding sites near BAT genes, WAT genes and control genes (genes with invariant expression between BAT and WAT, N = 2000 genes). The definition of BAT- and WAT- selective genes (N = 549 and N = 849, respectively) are shown in the methods. To the right, distance from NFI binding sites to the nearest DR1 motif is shown for binding sites near BAT genes, WAT genes and control genes. Statistical significance was determined by Mann–Whitney U test. The analyses were performed once based on the ChIP-seq dataset.
Supplementary Figure 4 Co-localization of NFIA facilitates PPARγ binding even before differentiation.
(a,b) ChIP-qPCR analysis of NFIA (a) and PPARγ (b) in C2C12 cells with introduction of PPARγ alone or both PPARγ and NFIA, at day 0 of differentiation. Cidea 29k, Ppara 21k, Ppargc1a-97k, and Ucp1 9.5k are background sites. The representative result of two independent experiments is shown (N = 2 independent samples; mean ± S.E.M.). (c,d) ChIP-qPCR analysis of NFIA (c) and PPARγ (d) in C2C12 cells with or without introduction of NFIA, differentiation day 7. Cidea 29k, Ppara 21k, Ppargc1a-97k, and Ucp1 9.5k are background sites. The representative result of two independent experiments is shown (N = 2 independent samples; mean ± S.E.M.).
Supplementary Figure 5 Deficiency of NFIA results in decreased expression of the brown-fat-specific genes and reciprocal elevation of muscle genes.
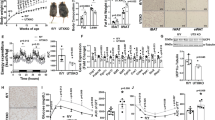
(a) RT-qPCR analysis Nfia and Pparg in WT, NFIA +/−, and NFIA −/− BAT (mean ± S.E.M.; N = 11 mice for WT, 24 mice for NFIA +/−, and 15 mice for NFIA −/−, respectively; ∗ p < 0.05, ∗∗ p < 0.01). (b) ChIP-qPCR analysis of PPARγ in brown adipocytes with NFIA knockdown. Ucp1 9.5k is a background site. The representative result of three independent experiments is shown (N = 2 independent samples; mean ± S.E.M.). (c–f) RNA-seq analysis of representative BAT genes (c), common genes (d), mitochondrial genes (e) and SKM genes (f) (mean ± S.E.M.; N = 3 independent samples, ∗ p < 0.05, ∗∗ p < 0.01). (g) RT-qPCR analysis of Nfia and Ucp1 in C57BL/6J and db/db mice (mean ± S.E.M.; N = 5 mice per group; ∗ p < 0.05, ∗∗ p < 0.01). The representative result of two independent experiments is shown.
Supplementary Figure 6 PPARγ is indispensable, while PGC1α and Adrb3 are dispensable for the effect of NFIA.
(a) Control sh RNA or sh RNA for PPARγ was introduced into control or NFIA-expressing C2C12 myoblasts, and stained with Oil Red O seven days after inducing adipocyte differentiation. Scale bar, 50 μm. (b–d) Pparg and Nfia (b), general adipocyte marker Fabp4 (c) and the brown-fat-specific genes Cidea and Ucp1 (d) were quantified by RT-qPCR at the indicated time course (mean ± S.E.M.; N = 3 independent samples; ∗ p < 0.05, ∗∗ p < 0.01). (e) Control si RNA or si RNA for PGC1α was introduced into control or NFIA-expressing C2C12 myoblasts, and stained with Oil Red O seven days after inducing adipocyte differentiation. Scale bar, 50 μm. (f–h) Ppargc1a and Nfia (f), common adipocyte genes (g) and the brown-fat-specific genes (h) were quantified by RT-qPCR at the indicated time course (mean ± S.E.M.; N = 3 independent samples; ∗ p < 0.05, ∗∗ p < 0.01). The representative result of two independent experiments is shown. (i) Control si RNA or si RNA for Adrb3 was introduced into control or NFIA-expressing C2C12 myoblasts, and stained with Oil Red O seven days after inducing adipocyte differentiation. Scale bar, 50 μm. (j–l) Adrb3 and Nfia (j), common adipocyte genes (k) and the brown-fat-specific genes (l) were quantified by RT-qPCR at the indicated time course (mean ± S.E.M.; N = 3 independent samples; ∗ p < 0.05, ∗∗ p < 0.01). The representative result of two independent experiments is shown.
Supplementary Figure 7 Overlap of NFI binding sites and BAT or eWAT FAIRE.
(a) Venn diagram showing the overlap of NFI binding sites in brown adipocytes, FAIRE peaks in BAT and FAIRE peaks in eWAT. (b) Bar graph showing the percentage of NFI binding sites that overlap only with BAT FAIRE peaks or eWAT FAIRE peaks. The analyses were performed once based on the ChIP-seq and FAIRE-seq dataset.
Supplementary information
Supplementary Information
Supplementary Information (PDF 3647 kb)
Supplementary Information
Life Sciences Reporting Summary
Supplementary Table 1
Supplementary Information
Supplementary Table 2
Supplementary Information
Rights and permissions
About this article
Cite this article
Hiraike, Y., Waki, H., Yu, J. et al. NFIA co-localizes with PPARγ and transcriptionally controls the brown fat gene program. Nat Cell Biol 19, 1081–1092 (2017). https://doi.org/10.1038/ncb3590
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb3590
This article is cited by
-
M2 macrophages independently promote beige adipogenesis via blocking adipocyte Ets1
Nature Communications (2024)
-
Adipogenic and SWAT cells separate from a common progenitor in human brown and white adipose depots
Nature Metabolism (2023)
-
Identification and characterization of the long non-coding RNA NFIA-AS2 as a novel locus for body mass index in American Indians
International Journal of Obesity (2023)
-
Microscopic image-based classification of adipocyte differentiation by machine learning
Histochemistry and Cell Biology (2023)
-
Browning of the white adipose tissue regulation: new insights into nutritional and metabolic relevance in health and diseases
Nutrition & Metabolism (2022)