Abstract
The PI(3)K–Akt–mTORC1 pathway is a highly dynamic network that is balanced and stabilized by a number of feedback inhibition loops1,2. Specifically, activation of mTORC1 has been shown to lead to the inhibition of its upstream growth factor signalling. Activation of the growth factor receptors is triggered by the binding of their cognate ligands in the extracellular space. However, whether secreted proteins contribute to the mTORC1-dependent feedback loops remains unclear. We found that cells with hyperactive mTORC1 secrete a protein that potently inhibits the function of IGF-1. Using a large-scale, unbiased quantitative proteomic platform, we comprehensively characterized the rapamycin-sensitive secretome in TSC2−/− mouse embryonic fibroblasts, and identified IGFBP5 as a secreted, mTORC1 downstream effector protein. IGFBP5 is a direct transcriptional target of HIF1, which itself is a known mTORC1 target3. IGFBP5 is a potent inhibitor of both the signalling and functional outputs of IGF-1. Once secreted, IGFBP5 cooperates with intracellular branches of the feedback mechanisms to block the activation of IGF-1 signalling. Finally, IGFBP5 is a potential tumour suppressor, and the proliferation of IGFBP5-mutated cancer cells is selectively blocked by IGF-1R inhibitors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Dibble, C. C. & Manning, B. D. Signal integration by mTORC1 coordinates nutrient input with biosynthetic output. Nat. Cell Biol. 15, 555–564 (2013).
Laplante, M. & Sabatini, D. M. mTOR signalling in growth control and disease. Cell 149, 274–293 (2012).
Wouters, B. G. & Koritzinsky, M. Hypoxia signalling through mTOR and the unfolded protein response in cancer. Nat. Rev. Cancer 8, 851–864 (2008).
Sancak, Y. et al. The Rag GTPases bind raptor and mediate amino acid signalling to mTORC1. Science 320, 1496–1501 (2008).
Ma, X. M. & Blenis, J. Molecular mechanisms of mTOR-mediated translational control. Nat. Rev. Mol. Cell Biol. 10, 307–318 (2009).
Hsu, P. P. et al. The mTOR-regulated phosphoproteome reveals a mechanism of mTORC1-mediated inhibition of growth factor signalling. Science 332, 1317–1322 (2011).
Robitaille, A. M. et al. Quantitative phosphoproteomics reveal mTORC1 activates de novo pyrimidine synthesis. Science 339, 1320–1323 (2013).
Yu, Y. et al. Phosphoproteomic analysis identifies Grb10 as an mTORC1 substrate that negatively regulates insulin signalling. Science 332, 1322–1326 (2011).
Shimobayashi, M. & Hall, M. N. Making new contacts: the mTOR network in metabolism and signalling crosstalk. Nat. Rev. Mol. Cell Biol. 15, 155–162 (2014).
Wagle, N. et al. Activating mTOR mutations in a patient with an extraordinary response on a phase I trial of everolimus and pazopanib. Cancer Discov. 4, 546–553 (2014).
Bissler, J. J. et al. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N. Engl. J. Med. 358, 140–151 (2008).
Wagle, N. et al. Response and acquired resistance to everolimus in anaplastic thyroid cancer. N. Engl. J. Med. 371, 1426–1433 (2014).
Cloughesy, T. F. et al. Antitumor activity of rapamycin in a Phase I trial for patients with recurrent PTEN-deficient glioblastoma. PLoS Med. 5, e8 (2008).
Chandarlapaty, S. et al. AKT inhibition relieves feedback suppression of receptor tyrosine kinase expression and activity. Cancer Cell 19, 58–71 (2011).
Zhang, H. et al. PDGFRs are critical for PI3K/Akt activation and negatively regulated by mTOR. J. Clin. Invest. 117, 730–738 (2007).
Harrington, L. S. et al. The TSC1-2 tumor suppressor controls insulin-PI3K signalling via regulation of IRS proteins. J. Cell Biol. 166, 213–223 (2004).
Shah, O. J. & Hunter, T. Turnover of the active fraction of IRS1 involves raptor-mTOR- and S6K1-dependent serine phosphorylation in cell culture models of tuberous sclerosis. Mol. Cell. Biol. 26, 6425–6434 (2006).
Liu, P. et al. Sin1 phosphorylation impairs mTORC2 complex integrity and inhibits downstream Akt signalling to suppress tumorigenesis. Nat. Cell Biol. 15, 1340–1350 (2013).
Yang, G., Murashige, D. S., Humphrey, S. J. & James, D. E. A positive feedback loop between Akt and mTORC2 via SIN1 phosphorylation. Cell Rep. 12, 937–943 (2015).
Bhaskar, P. T. et al. mTORC1 hyperactivity inhibits serum deprivation-induced apoptosis via increased hexokinase II and GLUT1 expression, sustained Mcl-1 expression, and glycogen synthase kinase 3β inhibition. Mol. Cell. Biol. 29, 5136–5147 (2009).
Makridakis, M. & Vlahou, A. Secretome proteomics for discovery of cancer biomarkers. J. Proteomics 73, 2291–2305 (2010).
Zhang, Y., Wang, J., Ding, M. & Yu, Y. Site-specific characterization of the Asp- and Glu-ADP-ribosylated proteome. Nat. Methods 10, 981–984 (2013).
Cornu, M. et al. Hepatic mTORC1 controls locomotor activity, body temperature, and lipid metabolism through FGF21. Proc. Natl Acad. Sci. USA 111, 11592–11599 (2014).
Hwa, V., Oh, Y. & Rosenfeld, R. G. The insulin-like growth factor-binding protein (IGFBP) superfamily. Endocr. Rev. 20, 761–787 (1999).
Cybulski, N., Polak, P., Auwerx, J., Ruegg, M. A. & Hall, M. N. mTOR complex 2 in adipose tissue negatively controls whole-body growth. Proc. Natl Acad. Sci. USA 106, 9902–9907 (2009).
Pearce, L. R. et al. Characterization of PF-4708671, a novel and highly specific inhibitor of p70 ribosomal S6 kinase (S6K1). Biochem. J. 431, 245–255 (2010).
Duvel, K. et al. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol. Cell 39, 171–183 (2010).
Li, S., Brown, M. S. & Goldstein, J. L. Bifurcation of insulin signalling pathway in rat liver: mTORC1 required for stimulation of lipogenesis, but not inhibition of gluconeogenesis. Proc. Natl Acad. Sci. USA 107, 3441–3446 (2010).
Owen, J. L. et al. Insulin stimulation of SREBP-1c processing in transgenic rat hepatocytes requires p70 S6-kinase. Proc. Natl Acad. Sci. USA 109, 16184–16189 (2012).
Semenza, G. L. Targeting HIF-1 for cancer therapy. Nat. Rev. Cancer 3, 721–732 (2003).
Semenza, G. L. et al. Hypoxia response elements in the aldolase A, enolase 1, and lactate dehydrogenase A gene promoters contain essential binding sites for hypoxia-inducible factor 1. J. Biol. Chem. 271, 32529–32537 (1996).
Salih, D. A. et al. Insulin-like growth factor-binding protein 5 (Igfbp5) compromises survival, growth, muscle development, and fertility in mice. Proc. Natl Acad. Sci. USA 101, 4314–4319 (2004).
Miyake, H., Pollak, M. & Gleave, M. E. Castration-induced up-regulation of insulin-like growth factor binding protein-5 potentiates insulin-like growth factor-I activity and accelerates progression to androgen independence in prostate cancer models. Cancer Res. 60, 3058–3064 (2000).
Carboni, J. M. et al. BMS-754807, a small molecule inhibitor of insulin-like growth factor-1R/IR. Mol. Cancer Ther. 8, 3341–3349 (2009).
Chen, H. X. & Sharon, E. IGF-1R as an anti-cancer target-trials and tribulations. Chin. J. Cancer 32, 242–252 (2013).
Mendel, D. B. et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin. Cancer Res. 9, 327–337 (2003).
Villen, J., Beausoleil, S. A., Gerber, S. A. & Gygi, S. P. Large-scale phosphorylation analysis of mouse liver. Proc. Natl Acad. Sci. USA 104, 1488–1493 (2007).
Olsen, J. V. et al. A dual pressure linear ion trap Orbitrap instrument with very high sequencing speed. Mol. Cell. Proteomics 8, 2759–2769 (2009).
Huttlin, E. L. et al. A tissue-specific atlas of mouse protein phosphorylation and expression. Cell 143, 1174–1189 (2010).
Ding, M., Bruick, R. & Yu, Y. Secreted IGFBP5 mediates mTORC-1-dependent feedback inhibition of IGF1 signaling. Protocol Exchange (2015)10.1038/protex.2015.124.
Nanjappa, V. et al. Plasma Proteome Database as a resource for proteomics research: 2014 update. Nucleic Acids Res. 42, D959–D965 (2014).
Maira, S. M. et al. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol. Cancer Ther. 7, 1851–1863 (2008).
Guo, Y. et al. TSC1 involvement in bladder cancer: diverse effects and therapeutic implications. J. Pathol. 230, 17–27 (2013).
Jehle, P. M., Jehle, D. R., Mohan, S. & Bohm, B. O. Serum levels of insulin-like growth factor system components and relationship to bone metabolism in Type 1 and Type 2 diabetes mellitus patients. J. Endocrinol. 159, 297–306 (1998).
Kong, S. E., Baxter, R. C. & Delhanty, P. J. Age-dependent regulation of the acid-labile subunit in response to fasting-refeeding in rats. Endocrinology 143, 4505–4512 (2002).
Acknowledgements
We thank J. Goldstein, J. Blenis, M. White, M. Cobb and B. Tu for critically reviewing the manuscript. We thank J. Minna and D. Kwiatkowski for providing critical reagents, and N. Williams, H. Wang, H. Chen and L. Wu for input and technical guidance. This work was supported in part by grants from the UT Southwestern Endowed Scholar Program (to Y.Y. and R.K.B.), the Cancer Prevention and Research Institute of Texas (CPRIT R1103 to Y.Y. and CPRIT RP130513 to R.K.B.), the Welch Foundation (I-1800 to Y.Y. and I-1568 to R.K.B.), National Institutes of Health (NIH; GM114160 to Y.Y.), American Cancer Society (Research Scholar Grant, RSG-15-062-01-TBE, and Institutional Research Grant, IRG-02-196-07, to Y.Y.), and a Career Award in the Biomedical Sciences from the Burroughs Wellcome Fund (to R.K.B.). Y.Y. is a Virginia Murchison Linthicum Scholar in Medical Research and a CPRIT Scholar in Cancer Research. R.K.B. is the Michael L. Rosenberg Scholar in Medical Research.
Author information
Authors and Affiliations
Contributions
Y.Y. conceived the project. M.D., R.K.B. and Y.Y. designed the experiments. M.D. and Y.Y. performed the experiments. R.K.B. provided input on HIF1-related experiments. Y.Y. wrote the manuscript with input from all co-authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1
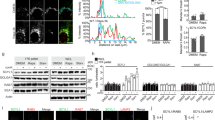
(A) LDH activity in the whole cell lysates and conditioned media from TSC2−/− MEFs was quantified using a coupled enzymatic colorimetric assay. LDH is a cytosolic enzyme that is found in nearly all living cells. (B) Chronic rapamcyin treatment (20 nM) of TSC2−/− MEFs led to Akt activation. For site-specific phosphorylation, pAkt(S473), pS6K(T389) and pS6(S235/236) levels were analyzed. (C) Heat-inactivated CM from TSC2−/− MEFs lost its ability to inhibit IGF-1 signalling. CM was collected from TSC2−/− MEFs and was heated at 95 °C for 5 min. CM was then mixed with IGF-1 (40 ng ml−1) and added to wt MEFs, the WCL of which were analyzed. For site-specific phosphorylation, pIGF-1R(Y1135/1136) and pAkt(S473) levels were analyzed. (D) Proteins identified from the two SILAC experiments. (E) Identification of FGF21 as a downstream target of mTORC1 in the extracellular space. Extracted ion chromatogram is shown for the corresponding light (rapamycin-treated, blue) and heavy (control, yellow) ions of a peptide (ALKPGVIQILGVK) from FGF21. This peptide showed a dramatic decrease after rapamycin treatment. (F) Identification of an IGFBP5 peptide (HMEASLQEFK). The MS2 spectrum from which the peptide was identified (matched b- and y- ions are highlighted in blue and red, respectively) is shown. (G) Re-introducing TSC2 into TSC2−/− MEFs led to dramatic downregulation of IGFBP5 in CM. For site-specific phosphorylation, pAkt(S473) levels were analyzed. (H) Rapamycin treatment also downregulated the expression of IGFBP5 in RT-4 cells. RT-4 cells were starved for 24 h, during which cells were treated with 20 nM rapamycin. For site-specific phosphorylation, pS6K(T389) and pS6(S235/236) levels were analyzed. (I) Insulin stimulation of MCF7 cells resulted in the accumulation of IGFBP5 in CM, which was blocked by concurrent rapamycin treatment. Cells were starved for 12 h, and were then treated with insulin (100 nM) for 12 h, in the absence or presence of rapamycin (20 nM). For site-specific phosphorylation, pAkt(S473) and pS6(S235/236) levels were analyzed.
Supplementary Figure 2 Gene ontology analysis of the rapamycin-sensitive proteins (abundances decreased by at least 32-fold after rapamycin treatment).
(A) Cellular compartment analysis. (B) Molecular function analysis. For clear presentation, only results from SILAC experiment #1 were considered.
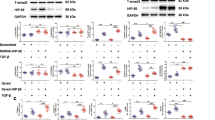
Supplementary Figure 3 A schematic indicating the possible model of HIF1-regulated expression of IGFBP5.
The design of various primers (P1-P4) capturing potential HIF1α binding sites on IGFBP5 is shown.
Supplementary Figure 4
(A) Proteins secreted from TSC2−/− MEFs inhibits the growth of MCF7 cells in a co-culture system. MCF7 cells were labeled with red florescent protein (DsRed), and were grown with GFP-labeled either TSC2+/+ or TSC2−/− MEFs. Cells were grown in DMEM supplemented with IGF-1. Scale bars, 40 μm. IGF-1 protects cells from staurosporine, (B), or etoposide (C)-induced apoptosis. This effect was abolished when IGFBP5 was co-added to the culture media. The asterisk indicates cleaved PARP1. For site-specific phosphorylation, pIGF-1R(Y1135/1136) and pAkt(S473) levels were analyzed. (D) IGFBP5 accounts for a major fraction of the IGF-1-inhibitory activity in CM from TSC2−/− MEFs. CM from TSC2+/+, shGFP TSC2−/− or shIGFBP5 TSC2−/− cells were mixed with IGF-1, and were added to recipient cells (wild type MEFs). WCL were analyzed by immunoblotting experiments using the indicated antibodies. The results were quantified using ImageJ. For site-specific phosphorylation, pIGF-1R(Y1135/1136) and pAkt(S473) levels were analyzed. *P < 0.05 (two-tailed Student t-test), NS = Not significant, n = 3 independent biological replicate experiments. Error bars represent s.d. (E) IGFBP5 mediates the mTORC1-dependent IGF-1-inhibitory activity in TSC2−/− MEFs. RNAi was used to knock down IGFBP5 in TSC2−/− MEFs. Where indicated, control knock down cells (shGFP TSC2−/− MEFs) were treated with rapamycin (20 nM for 24 h). The results were quantified using ImageJ. For site-specific phosphorylation, pIGF-1R(Y1135/1136), pAkt(S473) and pS6K(T389) levels were analyzed. pIGF-1R levels were normalized using total IGF-1R levels (note the rapamycin treatment increases total IGF1-R levels). **P < 0.01 (two-tailed Student t-test), NS = Not significant. n = 4 independent biological replicate experiments. Error bars represent s.d.
Supplementary Figure 5
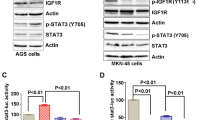
(A) CM from NCI-H1435 cells does not contain detectable IGFBP5 signals. CM from HEK293T cells that ectopically express IGFBP5 is used as the control. (B) Molt-4 cells (an acute lymphoblastic leukemia cell line that harbors a K135fs*13 mutation of the IGFBP5 gene) is more sensitive to IGF-1R inhibitors (BMS-536924 and BMS-754807), compared to Gefitinib and Sunitinib (48 h treatment). P < 0.001 (two-way ANOVA test). n = 9 independent biological replicate experiments. Error bars represent s.d. (C) The expression of wt-IGFBP5 in Molt-4 cells (to a level similar to that in IGFBP5-wt cells, e.g. T47D and RT-4) leads to reduced proliferation of these cells (grown in DMEM supplemented with 10% FBS). BMS-536924 treatment (500 nM) was used as the control. (D) The proliferation of IGFBP5-mutated (NCI-H1435), but not IGFBP5-wt (A549, NCI-H1693, HCC4017 and HCC15) NSCLC cells is sensitive to IGF-1R inhibitors (BMS-536924 and BMS-754807). None of these cells were sensitive to Sunitinib, a multi-RTK inhibitor, which, however, does not block IGF-1R.
Supplementary information
Supplementary Information
Supplementary Information (PDF 4398 kb)
Supplementary Table 1
Supplementary Information (XLSX 10 kb)
Supplementary Table 2
Supplementary Information (XLSX 9 kb)
Supplementary Table 3
Supplementary Information (XLSX 8 kb)
Supplementary Table 4
Supplementary Information (XLSX 64868 kb)
Supplementary Table 5
Supplementary Information (XLSX 85 kb)
Supplementary Table 6
Supplementary Information (XLSX 351 kb)
Supplementary Table 7
Supplementary Information (XLSX 352 kb)
Supplementary Table 8
Supplementary Information (XLSX 9 kb)
Supplementary Table 9
Supplementary Information (XLSX 9 kb)
Rights and permissions
About this article
Cite this article
Ding, M., Bruick, R. & Yu, Y. Secreted IGFBP5 mediates mTORC1-dependent feedback inhibition of IGF-1 signalling. Nat Cell Biol 18, 319–327 (2016). https://doi.org/10.1038/ncb3311
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb3311
This article is cited by
-
Alpha-linolenic acid modulates systemic and adipose tissue-specific insulin sensitivity, inflammation, and the endocannabinoid system in dairy cows
Scientific Reports (2023)
-
Insulin-like growth factor 5 associates with human Aß plaques and promotes cognitive impairment
Acta Neuropathologica Communications (2022)
-
Arctiin-reinforced antioxidant microcarrier antagonizes osteoarthritis progression
Journal of Nanobiotechnology (2022)
-
Quantitative phosphoproteomic analyses identify STK11IP as a lysosome-specific substrate of mTORC1 that regulates lysosomal acidification
Nature Communications (2022)
-
Proteomics-based prognostic signature and nomogram construction of hypoxia microenvironment on deteriorating glioblastoma (GBM) pathogenesis
Scientific Reports (2021)