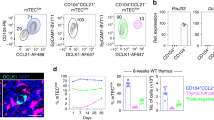
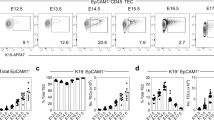
Abstract
Thymic T cell development is initiated from bone-marrow-derived multi potent thymus-seeding progenitors. During the early stages of thymocyte differentiation, progenitors become T cell restricted. However, the cellular environments supporting these critical initial stages of T cell development within the thymic cortex are not known. Here we use the dependence of early, c-Kit-expressing thymic progenitors on Kit ligand (KitL) to show that CD4−CD8−c-Kit+CD25− DN1-stage progenitors associate with, and depend on, the membrane-bound form of KitL (mKitL) provided by a cortex-specific KitL-expressing vascular endothelial cell (VEC) population. In contrast, the subsequent CD4−CD8−c-Kit+CD25+ DN2-stage progenitors associate selectively with cortical thymic epithelial cells (cTECs) and depend on cTEC-presented mKitL. These results show that the dynamic process of early thymic progenitor differentiation is paralleled by migration-dependent change to the supporting niche, and identify VECs as a thymic niche cell, with mKitL as a critical ligand.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Scadden, D. T. Nice neighborhood: emerging concepts of the stem cell niche. Cell 157, 41–50 (2014).
Scadden, D. T. The stem-cell niche as an entity of action. Nature 441, 1075–1079 (2006).
Chasis, J. A. & Mohandas, N. Erythroblastic islands: niches for erythropoiesis. Blood 112, 470–478 (2008).
Zhu, J. et al. Osteoblasts support B-lymphocyte commitment and differentiation from hematopoietic stem cells. Blood 109, 3706–3712 (2007).
Ding, L. & Morrison, S. J. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature 495, 231–235 (2013).
Bunting, M. D., Comerford, I. & McColl, S. R. Finding their niche: chemokines directing cell migration in the thymus. Immunol. Cell Biol. 89, 185–196 (2011).
Love, P. E. & Bhandoola, A. Signal integration and crosstalk during thymocyte migration and emigration. Nat. Rev. Immunol. 11, 469–477 (2011).
Radtke, F. et al. Deficient T cell fate specification in mice with an induced inactivation of Notch1. Immunity 10, 547–558 (1999).
Koch, U. et al. Delta-like 4 is the essential, nonredundant ligand for Notch1 during thymic T cell lineage commitment. J. Exp. Med. 205, 2515–2523 (2008).
Von Freeden-Jeffry, U., Solvason, N., Howard, M. & Murray, R. The earliest T lineage-committed cells depend on IL-7 for Bcl-2 expression and normal cell cycle progression. Immunity 7, 147–154 (1997).
Sitnicka, E. et al. Critical role of FLT3 ligand in IL-7 receptor independent T lymphopoiesis and regulation of lymphoid-primed multipotent progenitors. Blood 110, 2955–2964 (2007).
Kwan, J. & Killeen, N. CCR7 directs the migration of thymocytes into the thymic medulla. J. Immunol. 172, 3999–4007 (2004).
Trampont, P. C. et al. CXCR4 acts as a costimulator during thymic β-selection. Nat. Immunol. 11, 162–170 (2010).
Shah, D. K. & Zuniga-Pflucker, J. C. An overview of the intrathymic intricacies of T cell development. J. Immunol. 192, 4017–4023 (2014).
Tsai, P. T., Lee, R. A. & Wu, H. BMP4 acts upstream of FGF in modulating thymic stroma and regulating thymopoiesis. Blood 102, 3947–3953 (2003).
Heinonen, K. M. et al. Wnt4 regulates thymic cellularity through the expansion of thymic epithelial cells and early thymic progenitors. Blood 118, 5163–5173 (2011).
Hager-Theodorides, A. L. et al. Bone morphogenetic protein 2/4 signaling regulates early thymocyte differentiation. J. Immunol. 169, 5496–5504 (2002).
Takahama, Y. Journey through the thymus: stromal guides for T-cell development and selection. Nat. Rev. Immunol. 6, 127–135 (2006).
Rodewald, H. R., Kretzschmar, K., Swat, W. & Takeda, S. Intrathymically expressed c-kit ligand (stem cell factor) is a major factor driving expansion of very immature thymocytes in vivo. Immunity 3, 313–319 (1995).
Lennartsson, J. & Ronnstrand, L. Stem cell factor receptor/c-Kit: from basic science to clinical implications. Phys. Rev. 92, 1619–1649 (2012).
Gray, D. H., Chidgey, A. P. & Boyd, R. L. Analysis of thymic stromal cell populations using flow cytometry. J. Immunol. Methods 260, 15–28 (2002).
Red-Horse, K., Ueno, H., Weissman, I. L. & Krasnow, M. A. Coronary arteries form by developmental reprogramming of venous cells. Nature 464, 549–553 (2010).
Brannan, C. I. et al. Steel-Dickie mutation encodes a c-kit ligand lacking transmembrane and cytoplasmic domains. Proc. Natl Acad. Sci. USA 88, 4671–4674 (1991).
Flanagan, J. G., Chan, D. C. & Leder, P. Transmembrane form of the kit ligand growth factor is determined by alternative splicing and is missing in the Sld mutant. Cell 64, 1025–1035 (1991).
Gu, Y., Runyan, C., Shoemaker, A., Surani, M. A. & Wylie, C. Membrane-bound Steel factor maintains a high local concentration for mouse primordial germ cell motility, and defines the region of their migration. PLoS ONE 6, e25984 (2011).
Mikkelsen, H. B., Malysz, J., Huizinga, J. D. & Thuneberg, L. Action potential generation, Kit receptor immunohistochemistry and morphology of Steel-Dickie (Sl/Sld) mutant mouse small intestine. Neurogastroenterol. Motil. 10, 11–26 (1998).
Kapur, R. et al. Signaling through the interaction of membrane-restricted stem cell factor and c-kit receptor tyrosine kinase: genetic evidence for a differential role in erythropoiesis. Blood 91, 879–889 (1998).
Russell, E. S. Hereditary anemias of the mouse: a review for geneticists. Adv. Genet. 20, 357–459 (1979).
Kisanuki, Y. Y. et al. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev. Biol. 230, 230–242 (2001).
Claxton, S. et al. Efficient, inducible Cre-recombinase activation in vascular endothelium. Genesis 46, 74–80 (2008).
Srinivas, S. et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 1, 4 (2001).
Gordon, J. et al. Specific expression of lacZ and cre recombinase in fetal thymic epithelial cells by multiplex gene targeting at the Foxn1 locus. BMC Dev. Biol. 7, 69 (2007).
Calderon, L. & Boehm, T. Synergistic, context-dependent, and hierarchical functions of epithelial components in thymic microenvironments. Cell 149, 159–172 (2012).
Krause, D. S. & Scadden, D. T. Deconstructing the complexity of a microenvironmental niche. Cell 149, 16–17 (2012).
Ramasamy, S. K., Kusumbe, A. P. & Adams, R. H. Regulation of tissue morphogenesis by endothelial cell-derived signals. Trends Cell Biol. 25, 148–157 (2014).
Sasaki, T. et al. Regulation of hematopoietic cell clusters in the placental niche through SCF/Kit signaling in embryonic mouse. Development 137, 3941–3952 (2010).
Ding, L., Saunders, T. L., Enikolopov, G. & Morrison, S. J. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 481, 457–462 (2012).
Badea, T. C., Wang, Y. & Nathans, J. A noninvasive genetic/pharmacologic strategy for visualizing cell morphology and clonal relationships in the mouse. J. Neurosci. 23, 2314–2322 (2003).
Moreira, P. N., Pozueta, J., Giraldo, P., Gutierrez-Adan, A. & Montoliu, L. Generation of yeast artificial chromosome transgenic mice by intracytoplasmic sperm injection. Methods Mol. Biol. 349, 151–161 (2006).
Farley, F. W., Soriano, P., Steffen, L. S. & Dymecki, S. M. Widespread recombinase expression using FLPeR (flipper) mice. Genesis 28, 106–110 (2000).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Ramskold, D. et al. Full-length mRNA-Seq from single-cell levels of RNA and individual circulating tumor cells. Nat. Biotechnol. 30, 777–782 (2012).
Kim, D. et al. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 14, R36 (2013).
Grover, A. et al. Erythropoietin guides multipotent hematopoietic progenitor cells toward an erythroid fate. J. Exp. Med. 211, 181–188 (2014).
Acknowledgements
We thank C. Blackburn (Institute for Stem Cell Research, UK), J. Nathans (Johns Hopkins Medical School, Maryland, USA) and M. Fruttiger (National Institute of Medical Research, UK) for providing Foxn1–Cre, Rosa26–CreERT2 and Pdgfb–CreERT2 mice, respectively, A. Samraj for screening of targeted embryonic stem cells, and the Wolfson Imaging Centre for image analysis. This work was supported by an MRC Strategic Award (G0701761) and MRC Program Grant (G0900892) to C.N., by an MRC Program Grant to S.E.W.J., by Cancer Research UK and by Leukemia and Lymphoma Research. A.J.M. is the recipient of an MRC Senior Clinical fellowship.
Author information
Authors and Affiliations
Contributions
M.B. carried out mouse genetic experiments and imaging, and associated data analysis; R.F. and S.M. generated Sl/Sld andΔEx7 mice and measured KitL levels and activity; S.T. analysed RNASeq data; T.C.L. provided protocols for single-cell profiling; A.G. generated RNAseq libraries; D.W. performed image analysis; P.B. provided ICKitL-specific antibody; A.J.M. provided protocols for single-cell profiling; S.E.W.J. and C.N. conceived the project and wrote the manuscript with M.B.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 5 Heat maps of stromal cell gene expression.
(a) Heat map of gene expression data from Fig. 3a–g. RPKM values were normalized for each individual gene across all cell types. (b) mRNA expression in single KT+ VECs, KT− VECs (h) and cTECs and mTECs (i) using microfluidics-based qPCR. Expression values are normalized to B2m for each gene, and subsequently to the average value for each individual gene across all cell types. Each column represents a single cell.
Supplementary Figure 6 Lack of detectable sKitL expression from Steel-Dickie allele.
(a) Serum KItL levels in 3-week-old wild-type (+/+; n = 18), Sl/+ (n = 7), Sld/+ (n = 7) and Sl/Sld (n = 4) mice measured by ELISA. Error bars show standard errors. Student’s t-test P values are shown. For source data see Supplementary Table 5. (b) Schematic of the exon 7 floxed Kitl allele. (c) Concentration of sKItL in supernatants of HEK293 cells transfected with the empty MSCV-IRES-GFP vector (N = 6) or MSCV-IRES-GFP vectors encoding Sld KitL (N = 3) and ΔEx7 KitL (N = 3), respectively. Error bars show standard errors. For source data see Supplementary Table 5. (d) Bioassay measuring proliferation of the KitL-dependent MC/9 cell line exposed to the supernatants from (c). Values are relative to MC/9 cells grown under optimal proliferation conditions. Error bars show standard errors. N = 3 for each condition. For source data see Supplementary Table 5. (e) Serum KItL levels in 3 week old control (+/+; n = 6) and KitlΔEx7/ΔEx7 (n = 4) mice measured by ELISA. Error bars show standard errors. Student’s t-test P value is shown. For source data see Supplementary Table 5. (f) Total thymic cellularity of 4–5 weeks old +/+ mice (n = 3) and their Sl/+ littermates (n = 3). Error bars show standard errors. The difference between +/+ and Sl/+ genotypes is not statistically significant (P = 0.71). For source data see Supplementary Table 5. (g) Absolute number of phenotypically defined Flt3 + DN1, Flt3–DN1 and DN2 cells in thymi of mice from (f). Error bars show standard errors. No statistical significant differences were observed (P = 0.21, P = 0.48 and P = 0.35, respectively). For source data see Supplementary Table 5. (h) Total bone marrow cells in mice from (f). Error bars show standard errors. The difference between +/+ and Sl/+ is not statistically significant (P = 0.206). For source data see Supplementary Table 5. (i) Number of cells in the indicated phenotypically defined lymphoid progenitor populations in mice from (f). Values are group averages; error bars show standard errors. P-values relative to the control group are shown. For source data see Supplementary Table 5.
Supplementary Figure 7 Specificity of Cre lines used.
(a) Schematic of the Tie2-Cre lineage tracing using the Rosa26-EYFP Cre reporter. (b) Flow cytometric analysis of thymic stromal cell subsets from Tie2-Cre; Rosa26-EYFP mice (green lines) and Rosa26-EYFP control mice (blue lines). Percentage of YFP + cells observed in the VEC (right plot) and combined MCs and TECs populations (left plot) is shown. (c) Schematic of the Pdgfb − CreERT2-IRES-iGFP reporter. (d) Flow cytometric analysis of thymic stromal cell subsets from Pdgfb − CreERT2-IRES-iGFP mice (green lines) and non-transgenic control mice (blue lines). Percentage of GFP + cells detected in the VECs (right plot) and the combined MC and TEC populations (left plot) is shown. (e) Schematic of Foxn1-Cre lineage tracing using the Rosa26-EYFP Cre reporter. (f) Flow cytometry analysis of thymic stromal cell subsets in the Foxn1-Cre/Rosa26-EYFP mouse model. Percentage of EYFP + cells in the TEC (right plot) and MC plus VEC lineages (left plot) is displayed. (g) Total thymic cells from 6 weeks old controls (n = 3 biological replicates pooled over 2 independent experiments) and Pdgfb-CreERT2 transgenic (n = 3) littermates, treated with tamoxifen and analysed one week after the last injection. The Cre driver is shown below the x-axis. Values are group averages. Error bars show standard errors. No statistical significant differences were observed. For source data see Supplementary Table 5. (h) Absolute number of phenotypic Flt3 + DN1, Flt3- DN1 and DN2 thymocytes from the mice in (g). Error bars show standard errors. No statistical significant differences were observed. For source data see Supplementary Table 5. (i) Number of total bone marrow cells from the mice analysed in (g). Error bars show standard errors. No statistical significant differences were observed. For source data see Supplementary Table 5. (j) Absolute number of phenotypic bone marrow lymphoid progenitor populations from the mice in (g). Error bars show standard errors. No statistically significant differences were observed. For source data see Supplementary Table 5. (k) Absolute number of phenotypic DAPI−lineage−B220−CD4−CD8− (DN), DAPI−CD4 + CD8 + (DP), DAPI−CD4+CD8− (SP4), DAPI−CD4−CD8+ (SP8) from 4–5 weeks old uninjected (n = 6) and tamoxifen injected (n = 6) control mice pooled from 3 independent experiments. Error bars show standard errors. No statistically significant differences were observed.
Supplementary Figure 8 Loss of mKitL does not affect thymic architecture or morphology.
Immunofluorescence analysis of thymic sections by using antibodies against CD31 (green), Ly51 (red), UEA-1 (blue) to assess the overall thymus architecture in 4–5 weeks old control (a–d) (KitlLEx7/LEx7) and FoxN1ΔEx7 mice (e–h). Scale bar: 300 μm. Immunofluorescence analysis of thymic sections by using antibodies against CD31 (green), Ly51 (red), UEA-1 (blue) in 4–5 weeks old, tamoxifen injected control (i–l) (KitlLEx7/LEx7), Tie2/PdgfbΔEx7 mice (m–p) and Tie2/Pdgfb/FoxN1ΔEx7 mice. Scale bar: 300 μm.
Supplementary Figure 9 Impact of mKitL depletion on thymic stromal cell and thymic progenitor populations.
(a) Relative percentage of phenotypic TECs, VECs and MCs from 4–5 weeks old control, Foxn1ΔEx7 and Tie2/PdgfbΔEx7 mice analyzed as in Fig. 5a. Number of biological replicates displayed. Error bars show standard errors. No statistically significant differences were observed. For source data see Supplementary Table 5. (b) Absolute number of DN, DP, SP4 and SP8 from 4–5 week old control (n = 7) and Foxn1ΔEx7 mice (n = 3). Error bars show standard errors. P-values relative to the control group are shown. For source data see Supplementary Table 5. (c) Absolute number of phenotypic DN, DP, SP4 and SP8 from 4–5 weeks old control (n = 5), Tie2/PdgfbΔEx7 (n = 4) and Tie2/Pdgfb/FoxN1ΔEx7 (n = 5), analyzed as in Fig. 5a. Error bars show standard errors. P-values relative to the control group are shown. For source data see Supplementary Table 5.
Supplementary Figure 10 Effect of stromal mKitL deletion on bone marrow lymphopoiesis.
(a) Number of total bone marrow cells from KitlLEx7/LEx7 (n = 4) and Tie2/PdgfbΔEx7 (n = 4) mice from 2 separate experiments, treated with tamoxifen and analysed as in Fig. 5a. Error bars show standard errors. No statistically significant differences were observed. (b) Number of phenotypic bone marrow lymphoid progenitor populations in mice from (a). Error bars show standard errors. No statistically significant differences were observed. (c) Number of total bone marrow cells from KitlLEx7/LEx7 (n = 7) and Foxn1ΔEx7 (n = 7) mice from 3 separate experiments. Error bars show standard errors. P-values relative to the control group are shown (Student’s t-test). (d) Number of phenotypic bone marrow lymphoid progenitor populations in mice from (c). Error bars show standard errors. No statistically significant differences were observed. (e) Number of total bone marrow cells from KitlLEx7/LEx7 (n = 6) and Tie2/Pdgfb/Foxn1ΔEx7 (n = 5) mice analyzed as in (a) from 4 separate experiments. Error bars show standard errors. No statistically significant differences were observed. (f) Number of phenotypic bone marrow lymphoid progenitor populations in mice from (e). Error bars show standard errors. No statistically significant differences were observed.
Supplementary Figure 11 Thymic stromal cells FACS analysis.
Gating strategy used to define thymic stromal cell populations within the total thymic live (DAPI −) singlet cell population: VECs: Lin−EpCAM−CD31+; MCs Lin−EpCAM−CD31−,; cTECs: Lin−EpCAM+Ly51+UEA-1−; mTECs: Lin−CD31−EpCAM+Ly51−UEA-1+. Lin− is CD45−Ter119−.
Supplementary Figure 12 Thymic T cell progenitors and bone marrow lymphoid progenitors FACS analysis.
(a) Gating strategy used to define Flt3 + DN1 (Lin − CD4 − CD8α − CD25 − c-KithiFlt3 +), Flt3–DN1 (Lin − CD4 − CD8α − CD25 − c-KithiFlt3 −) and DN2 (Lin − CD4 − CD8α − CD25 + c-KithiFlt3 −) thymocytes in young adult mice (4–6 weeks). (b) Gating strategy used to define lymphoid progenitor populations within the bone marrow live (DAPI −) singlet cell population. The following populations were analysed: NK/T progenitors (LIn–B220 − CD49b − NK1.1 − CD122 +), Lymphoid-biased multi-potent progenitors (LMPPs: Lin − B220 − CD49b − NK1.1 − CD122 − Flt3 + CD127 − c-KithiSca1hi), All Lymphoid Progenitors (ALPs: Lin − B220 − CD49b − NK1.1 − CD122 − Flt3 + CD127 + Ly6D − c-Kitlo), B-Lymphoid Progenitors (BLPs: Lin − B220 − CD49b − NK1.1 − CD122 − Flt3 + CD127 + Ly6D + c-Kit −), Common Lymphoid Progenitors (CLPs: Lin − B220 − CD49b − NK1.1 − CD122 − Flt3 + CD127 + Ly6D − c-KitloSca1lo).
Supplementary information
Supplementary Information
Supplementary Information (PDF 1517 kb)
Rights and permissions
About this article
Cite this article
Buono, M., Facchini, R., Matsuoka, S. et al. A dynamic niche provides Kit ligand in a stage-specific manner to the earliest thymocyte progenitors. Nat Cell Biol 18, 157–167 (2016). https://doi.org/10.1038/ncb3299
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb3299
This article is cited by
-
The Proteostasis of Thymic Stromal Cells in Health and Diseases
The Protein Journal (2024)
-
Slow integrin-dependent migration organizes networks of tissue-resident mast cells
Nature Immunology (2023)
-
How transcription factors drive choice of the T cell fate
Nature Reviews Immunology (2021)
-
Bi-directional signaling by membrane-bound KitL induces proliferation and coordinates thymic endothelial cell and thymocyte expansion
Nature Communications (2018)
-
Thymus Colonization: Who, How, How Many?
Archivum Immunologiae et Therapiae Experimentalis (2018)