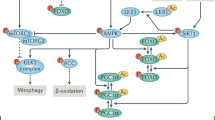
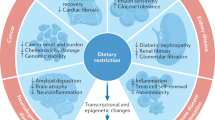
Abstract
A hallmark of ageing is dysfunction in nutrient signalling pathways that regulate glucose homeostasis, negatively affecting whole-body energy metabolism and ultimately increasing the organism's susceptibility to disease. Maintenance of insulin sensitivity depends on functional mitochondrial networks, but is compromised by alterations in mitochondrial energy metabolism during ageing. Here we discuss metabolic paradigms that influence mammalian longevity, and highlight recent advances in identifying fundamental signalling pathways that influence metabolic health and ageing through mitochondrial perturbations.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. The hallmarks of aging. Cell 153, 1194–1217 (2013).
De Cabo, R., Carmona-Gutierrez, D., Bernier, M., Hall, M. N. & Madeo, F. The search for antiaging interventions: from elixirs to fasting regimens. Cell 157, 1515–1526 (2014).
Selman, C. & Withers, D. J. Mammalian models of extended healthy lifespan. Philos. Trans. R. Soc. Lond. Ser. B. Biol. Sci. 366, 99–107 (2011).
Rowe, J. W., Minaker, K. L., Pallotta, J. A. & Flier, J. S. Characterization of the insulin resistance of aging. J. Clin. Invest. 71, 1581–1587 (1983).
Fink, R. I., Kolterman, O. G., Griffin, J. & Olefsky, J. M. Mechanisms of insulin resistance in aging. J. Clin. Invest. 71, 1523–1535 (1983).
Houtkooper, R. H. et al. The metabolic footprint of aging in mice. Sci. Rep. 1, 134 (2011).
Riera, C. E. et al. TRPV1 pain receptors regulate longevity and metabolism by neuropeptide signaling. Cell 157, 1023–1036 (2014).
Kurosu, H. et al. Suppression of aging in mice by the hormone klotho. Science 309, 1829–1833 (2005).
Selman, C. et al. Evidence for lifespan extension and delayed age-related biomarkers in insulin receptor substrate 1 null mice. FASEB J. 22, 807–818 (2008).
Taguchi, A., Wartschow, L. M. & White, M. F. Brain IRS2 signaling coordinates life span and nutrient homeostasis. Science 317, 369–372 (2007).
Speakman, J. R. et al. Uncoupled and surviving: individual mice with high metabolism have greater mitochondrial uncoupling and live longer. Aging Cell 3, 87–95 (2004).
Galgani, J. E., Moro, C. & Ravussin, E. Metabolic flexibility and insulin resistance. Am. J. Physiol. Endocrinol. Metab. 295, 1009–1017 (2008).
Kondratov, R. V., Kondratova, A. A., Gorbacheva, V. Y., Vykhovanets, O. V. & Antoch, M. P. Early aging and age-related pathologies in mice deficient in BMAL1, the core component of the circadian clock. Genes Dev. 20, 1868–1873 (2006).
Chang, H-C. & Guarente, L. SIRT1 mediates central circadian control in the SCN by a mechanism that decays with aging. Cell 153, 1448–1460 (2013).
Sahar, S. & Sassone-Corsi, P. Metabolism and cancer: the circadian clock connection. Nat. Rev. Cancer 9, 886–896 (2009).
Fontana, L., Partridge, L. & Longo, V. D. Dietary restriction, growth factors and aging: from yeast to humans. Science 328, 321–326 (2010).
Mair, W. & Dillin, A. Aging and survival: the genetics of life span extension by dietary restriction. Annu. Rev. Biochem. 77, 727–754 (2008).
Bruss, M. D., Khambatta, C. F., Ruby, M. A., Aggarwal, I. & Hellerstein, M. K. Calorie restriction increases fatty acid synthesis and whole body fat oxidation rates. Am. J. Physiol. Endocrinol. Metab. 298, E108–E116 (2010).
Bluher, M. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science 299, 572–574 (2003).
Foukas, L. C. et al. Long-term p110? PI3K inactivation exerts a beneficial effect on metabolism. EMBO Mol. Med. 5, 563–571 (2013).
Zhang, Y. et al. The starvation hormone, fibroblast growth factor-21, extends lifespan in mice. eLife 1, e00065 (2012).
Yuan, R. et al. Aging in inbred strains of mice: study design and interim report on median lifespans and circulating IGF1 levels. Aging Cell 8, 277–287 (2009).
Berryman, D. E., Christiansen, J. S., Johannsson, G., Thorner, M. O. & Kopchick, J. J. Role of the GH/IGF-1 axis in lifespan and healthspan: lessons from animal models. Growth Horm. IGF Res. 18, 455–471 (2008).
Hsieh, C-C., DeFord, J. H., Flurkey, K., Harrison, D. E. & Papaconstantinou, J. Implications for the insulin signaling pathway in Snell dwarf mouse longevity: a similarity with the C. elegans longevity paradigm. Mech. Ageing Dev. 123, 1229–1244 (2002).
Dominici, F. P., Hauck, S., Argentino, D. P., Bartke, A. & Turyn, D. Increased insulin sensitivity and upregulation of insulin receptor, insulin receptor substrate (IRS)-1 and IRS-2 in liver of Ames dwarf mice. J. Endocrinol. 173, 81–94 (2002).
Shah, J. H. & Cerchio, G. M. Hypoinsulinemia of hypothyroidism. Arch. Intern. Med. 132, 657–661 (1973).
Tsugane, S. & Inoue, M. Insulin resistance and cancer: epidemiological evidence. Cancer Sci. 101, 1073–1079 (2010).
Naderali, E. K., Ratcliffe, S. H. & Dale, M. C. Obesity and Alzheimer's disease: a link between body weight and cognitive function in old age. Am. J. Alzheimers Dis. Other Demen. 24, 445–449 (2009).
Ortega-Molina, A. et al. Pten positively regulates brown adipose function, energy expenditure, and longevity. Cell Metab. 15, 382–394 (2012).
Coschigano, K. T., Clemmons, D., Bellush, L. L. & Kopchick, J. J. Assessment of growth parameters and life span of GHR/BP gene-disrupted mice. Endocrinology 141, 2608–2613 (2000).
Ikeno, Y., Bronson, R. T., Hubbard, G. B., Lee, S. & Bartke, A. Delayed occurrence of fatal neoplastic diseases in ames dwarf mice: correlation to extended longevity. J. Gerontol. A. Biol. Sci. Med. Sci. 58, 291–296 (2003).
Ikeno, Y. et al. Reduced incidence and delayed occurrence of fatal neoplastic diseases in growth hormone receptor/binding protein knockout mice. J. Gerontol. A. Biol. Sci. Med. Sci. 64, 522–529 (2009).
Harrison, D. E. et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460, 392–395 (2009).
Miller, R. A. et al. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J. Gerontol. A. Biol. Sci. Med. Sci. 66A, 191–201 (2011).
Corradetti, M. N. & Guan, K-L. Upstream of the mammalian target of rapamycin: do all roads pass through mTOR? Oncogene 25, 6347–6360 (2006).
Lamming, D. W. et al. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science 335, 1638–1643 (2012).
Roy, J., Paquette, J-S., Fortin, J.-F. & Tremblay, M. J. The immunosuppressant rapamycin represses human immunodeficiency virus type 1 replication. Antimicrob. Agents Chemother. 46, 3447–3455 (2002).
Fang, Y. et al. Duration of rapamycin treatment has differential effects on metabolism in mice. Cell Metab. 17, 456–462 (2013).
Pyo, J-O. et al. Overexpression of Atg5 in mice activates autophagy and extends lifespan. Nat. Commun. 4, 2300 (2013).
Selman, C. et al. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science 326, 140–144 (2009).
Wu, J. J. et al. Increased mammalian lifespan and a segmental and tissue-specific slowing of aging after genetic reduction of mTOR expression. Cell Rep. 4, 913–920 (2013).
Saltiel, A. R. & Kahn, C. R. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 414, 799–806 (2001).
Bratic, A. & Larsson, N-G. The role of mitochondria in aging. J. Clin. Invest. 123, 951–957 (2013).
Schapira, A. H. V. Mitochondrial diseases. Lancet 379, 1825–1834 (2012).
Herbener, G. H. A morphometric study of age-dependent changes in mitochondrial population of mouse liver and heart. J. Gerontol. 31, 8–12 (1976).
Lanza, I. R. & Nair, K. S. Mitochondrial function as a determinant of life span. Pflüg. Arch. Eur. J. Physiol. 459, 277–289 (2010).
Conley, K. E., Jubrias, S. A. & Esselman, P. C. Oxidative capacity and ageing in human muscle. J. Physiol. 526, 203–210 (2000).
Lin, J. et al. Transcriptional co-activator PGC-1α drives the formation of slow-twitch muscle fibres. Nature 418, 797–801 (2002).
Puigserver, P. et al. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 92, 829–839 (1998).
Mootha, V. K. et al. PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat. Genet. 34, 267–273 (2003).
Patti, M. E. et al. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1. Proc. Natl Acad. Sci. USA 100, 8466–8471 (2003).
Wenz, T., Rossi, S. G., Rotundo, R. L., Spiegelman, B. M. & Moraes, C. T. Increased muscle PGC-1α expression protects from sarcopenia and metabolic disease during aging. Proc. Natl Acad. Sci. USA 106, 20405–20410 (2009).
Anderson, R. & Prolla, T. PGC-1α in aging and anti-aging interventions. Biochim. Biophys. Acta 1790, 1059–1066 (2009).
Austin, S. & St-Pierre, J. PGC1α and mitochondrial metabolism — emerging concepts and relevance in ageing and neurodegenerative disorders. J. Cell Sci. 125, 4963–4971 (2012).
Cantó, C. et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 458, 1056–1060 (2009).
Jäger, S., Handschin, C., St-Pierre, J. & Spiegelman, B. M. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1α. Proc. Natl Acad. Sci. USA 104, 12017–12022 (2007).
Narkar, V. A. et al. AMPK and PPARδ agonists are exercise mimetics. Cell 134, 405–415 (2008).
Viscomi, C. et al. In vivo correction of COX deficiency by activation of the AMPK/PGC-1α axis. Cell Metab. 14, 80–90 (2011).
Martin-Montalvo, A. et al. Metformin improves healthspan and lifespan in mice. Nat. Commun. 4, 2192 (2013).
Kalender, A. et al. Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent manner. Cell Metab. 11, 390–401 (2010).
Cantó, C. et al. Interdependence of AMPK and SIRT1 for metabolic adaptation to fasting and exercise in skeletal muscle. Cell Metab. 11, 213–219 (2010).
Price, N. L. et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 15, 675–690 (2012).
Nicholls, D. G., Bernson, V. S. M. & Heaton, G. M. in Effectors of Thermogenesis (eds. Girardier, L. & Seydoux, J.) 89–93 (Birkhäuser Basel, 1978).
Echtay, K. S. et al. Superoxide activates mitochondrial uncoupling proteins. Nature 415, 96–99 (2002).
Gates, A. C. et al. Respiratory uncoupling in skeletal muscle delays death and diminishes age-related disease. Cell Metab. 6, 497–505 (2007).
Nisoli, E. et al. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science 310, 314–317 (2005).
Lanza, I. R. et al. Chronic caloric restriction preserves mitochondrial function in senescence without increasing mitochondrial biogenesis. Cell Metab. 16, 777–788 (2012).
St-Pierre, J. et al. Bioenergetic analysis of peroxisome proliferator-activated receptor γ coactivators 1α and 1β (PGC-1α and PGC-1β) in muscle cells. J. Biol. Chem. 278, 26597–26603 (2003).
Schriner, S. E. et al. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science 308, 1909–1911 (2005).
Pérez, V. I. et al. The overexpression of major antioxidant enzymes does not extend the lifespan of mice. Aging Cell 8, 73–75 (2009).
Van Remmen, H. et al. Life-long reduction in MnSOD activity results in increased DNA damage and higher incidence of cancer but does not accelerate aging. Physiol. Genomics 16, 29–37 (2003).
Zhang, Y. et al. Mice deficient in both Mn superoxide dismutase and glutathione peroxidase-1 have increased oxidative damage and a greater incidence of pathology but no reduction in longevity. J. Gerontol. A. Biol. Sci. Med. Sci. 64, 1212–1220 (2009).
Edgar, D. et al. Random point mutations with major effects on protein-coding genes are the driving force behind premature aging in mtDNA mutator mice. Cell Metab. 10, 131–138 (2009).
Trifunovic, A. et al. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature 429, 417–423 (2004).
Vermulst, M. et al. DNA deletions and clonal mutations drive premature aging in mitochondrial mutator mice. Nat. Genet. 40, 392–394 (2008).
Kolesar, J. E. et al. Defects in mitochondrial DNA replication and oxidative damage in muscle of mtDNA mutator mice. Free Radic. Biol. Med. 75, 241–251 (2014).
Yee, C., Yang, W. & Hekimi, S. The intrinsic apoptosis pathway mediates the pro-longevity response to mitochondrial ROS in C. elegans. Cell 157, 897–909 (2014).
Dillin, A. et al. Rates of behavior and aging specified by mitochondrial function during development. Science 298, 2398–2401 (2002).
Lee, S. S. et al. A systematic RNAi screen identifies a critical role for mitochondria in C. elegans longevity. Nat. Genet. 33, 40–48 (2003).
Durieux, J., Wolff, S. & Dillin, A. The cell-non-autonomous nature of electron transport chain-mediated longevity. Cell 144, 79–91 (2011).
Yoneda, T. et al. Compartment-specific perturbation of protein handling activates genes encoding mitochondrial chaperones. J. Cell Sci. 117, 4055–4066 (2004).
Mottis, A., Jovaisaite, V. & Auwerx, J. The mitochondrial unfolded protein response in mammalian physiology. Mamm. Genome 25, 424–433 (2014).
Zhao, Q. et al. A mitochondrial specific stress response in mammalian cells. EMBO J. 21, 4411–4419 (2002).
Houtkooper, R. H. et al. Mitonuclear protein imbalance as a conserved longevity mechanism. Nature 497, 451–457 (2013).
Dell'agnello, C. et al. Increased longevity and refractoriness to Ca2+-dependent neurodegeneration in Surf1 knockout mice. Hum. Mol. Genet. 16, 431–444 (2007).
Lapointe, J. & Hekimi, S. Early mitochondrial dysfunction in long-lived Mclk1+/− mice. J. Biol. Chem. 283, 26217–26227 (2008).
Bai, P. et al. PARP-2 regulates SIRT1 expression and whole-body energy expenditure. Cell Metab. 13, 450–460 (2011).
Bai, P. et al. PARP-1 inhibition increases mitochondrial metabolism through SIRT1 activation. Cell Metab. 13, 461–468 (2011).
Lagouge, M. et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell 127, 1109–1122 (2006).
Yoshino, J., Mills, K. F., Yoon, M. J. & Imai, S. Nicotinamide mononucleotide, a key NAD+ intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab. 14, 528–536 (2011).
Kelley, D. E., He, J., Menshikova, E. V. & Ritov, V. B. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 51, 2944–2950 (2002).
Fisher-Wellman, K. H. & Neufer, P. D. Linking mitochondrial bioenergetics to insulin resistance via redox biology. Trends Endocrinol. Metab. 23, 142–153 (2012).
Lowell, B. B. & Shulman, G. I. Mitochondrial dysfunction and type 2 diabetes. Science 307, 384–387 (2005).
Cholerton, B., Baker, L. D. & Craft, S. Insulin resistance and pathological brain ageing. Diabet. Med. 28, 1463–1475 (2011).
Evans, J. L., Maddux, B. A. & Goldfine, I. D. The molecular basis for oxidative stress-induced insulin resistance. Antioxid. Redox Signal. 7, 1040–1052 (2005).
Halliwell, B. Reactive oxygen species and the central nervous system. J. Neurochem. 59, 1609–1623 (1992).
Pipatpiboon, N., Pratchayasakul, W., Chattipakorn, N. & Chattipakorn, S. C. PPARγ agonist improves neuronal insulin receptor function in hippocampus and brain mitochondria function in rats with insulin resistance induced by long term high-fat diets. Endocrinology 153, 329–338 (2012).
Thaler, J. P. et al. Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Invest. 122, 153–162 (2012).
Kleinridders, A. et al. Leptin regulation of Hsp60 impacts hypothalamic insulin signaling. J. Clin. Invest. 123, 4667–4680 (2013).
Chan, D. C. Fusion and fission: interlinked processes critical for mitochondrial health. Annu. Rev. Genet. 46, 265–287 (2012).
Gomes, L. C., Di Benedetto, G. & Scorrano, L. During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nat. Cell Biol. 13, 589–598 (2011).
Green, D. R., Galluzzi, L. & Kroemer, G. Mitochondria and the autophagy- inflammation-cell death axis in organismal aging. Science 333, 1109–1112 (2011).
Zhang, J. Autophagy and mitophagy in cellular damage control. Redox Biol. 1, 19–23 (2013).
Lee, S. et al. Mitochondrial fission and fusion mediators, hFis1 and OPA1, modulate cellular senescence. J. Biol. Chem. 282, 22977–22983 (2007).
Dietrich, M. O., Liu, Z-W. & Horvath, T. L. Mitochondrial dynamics controlled by mitofusins regulate Agrp neuronal activity and diet-induced obesity. Cell 155, 188–199 (2013).
Schneeberger, M. et al. Mitofusin 2 in POMC neurons connects ER stress with leptin resistance and energy imbalance. Cell 155, 172–187 (2013).
De Brito, O. M. & Scorrano, L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature 456, 605–610 (2008).
Sebastián, D. et al. Mitofusin 2 (Mfn2) links mitochondrial and endoplasmic reticulum function with insulin signaling and is essential for normal glucose homeostasis. Proc. Natl Acad. Sci. USA 109, 5523–5528 (2012).
Muñoz, J. P. et al. Mfn2 modulates the UPR and mitochondrial function via repression of PERK. EMBO J. 32, 2348–2361 (2013).
Ozcan, U. et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306, 457–461 (2004).
Garg, A. D. et al. ER stress-induced inflammation: does it aid or impede disease progression? Trends Mol. Med. 18, 589–598 (2012).
Zhang, K. & Kaufman, R. J. From endoplasmic-reticulum stress to the inflammatory response. Nature 454, 455–462 (2008).
Zhang, G. et al. Hypothalamic programming of systemic ageing involving IKK-β, NF-κB and GnRH. Nature 497, 211–216 (2013).
Cheng, Z. & Almeida, F. A. Mitochondrial alteration in type 2 diabetes and obesity: an epigenetic link. Cell Cycle 13, 890–897 (2014).
Acknowledgements
We apologize to our colleagues for omitting numerous references due to space limitations. We thank Carsten Merkwirth and Kristen Berendzen for their helpful comments on the manuscript. C.E.R is supported by the American Diabetes Association Pathway to Stop Diabetes Grant 1-15-INI-12.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Riera, C., Dillin, A. Tipping the metabolic scales towards increased longevity in mammals. Nat Cell Biol 17, 196–203 (2015). https://doi.org/10.1038/ncb3107
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb3107
This article is cited by
-
The metabolic contribution of SKN-1/Nrf2 to the lifespan of Caenorhabditis elegans
Metabolomics (2023)
-
The effect of glycine administration on the characteristics of physiological systems in human adults: A systematic review
GeroScience (2023)
-
Hesperetin promotes longevity and delays aging via activation of Cisd2 in naturally aged mice
Journal of Biomedical Science (2022)
-
Healthy aging and muscle function are positively associated with NAD+ abundance in humans
Nature Aging (2022)
-
Lacticaseibacillus rhamnosus Probio-M9 extends the lifespan of Caenorhabditis elegans
Communications Biology (2022)