Abstract
Insulin resistance is a cardinal feature of Type 2 diabetes (T2D) and a frequent complication of multiple clinical conditions, including obesity, ageing and steroid use, among others. How such a panoply of insults can result in a common phenotype is incompletely understood. Furthermore, very little is known about the transcriptional and epigenetic basis of this disorder, despite evidence that such pathways are likely to play a fundamental role. Here, we compare cell autonomous models of insulin resistance induced by the cytokine tumour necrosis factor-α or by the steroid dexamethasone to construct detailed transcriptional and epigenomic maps associated with cellular insulin resistance. These data predict that the glucocorticoid receptor and vitamin D receptor are common mediators of insulin resistance, which we validate using gain- and loss-of-function studies. These studies define a common transcriptional and epigenomic signature in cellular insulin resistance enabling the identification of pathogenic mechanisms.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Houstis, N., Rosen, E. D. & Lander, E. S. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 440, 944–948 (2006).
Hotamisligil, G. S. & Spiegelman, B. M. Tumor necrosis factor α: a key component of the obesity-diabetes link. Diabetes 43, 1271–1278 (1994).
van Raalte, D. H., Ouwens, D. M. & Diamant, M. Novel insights into glucocorticoid-mediated diabetogenic effects: towards expansion of therapeutic options? Eur. J. Clin. Invest. 39, 81–93 (2009).
Hoehn, K. L. et al. Insulin resistance is a cellular antioxidant defense mechanism. Proc. Natl Acad. Sci. USA 106, 17787–17792 (2009).
James, A. M., Collins, Y., Logan, A. & Murphy, M. P. Mitochondrial oxidative stress and the metabolic syndrome. Trends Endocrinol. Metab. 23, 429–434 (2012).
Glass, C. K. & Olefsky, J. M. Inflammation and lipid signaling in the etiology of insulin resistance. Cell Metab. 15, 635–645 (2012).
Hotamisligil, G. S. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 140, 900–917 (2010).
Tontonoz, P. & Spiegelman, B. M. Fat and beyond: the diverse biology of PPARγ. Annual Rev. Biochem. 77, 289–312 (2008).
Schulz, L. C. The Dutch Hunger Winter and the developmental origins of health and disease. Proc. Natl Acad. Sci. USA 107, 16757–16758 (2010).
Rinaudo, P. & Wang, E. Fetal programming and metabolic syndrome. Annual Rev. Phys. 74, 107–130 (2012).
Masuccio, F. et al. Weight gain and insulin resistance in children treated with valproate: the influence of time. J. Child Neurol. 25, 941–947 (2010).
Tateishi, K., Okada, Y., Kallin, E. M. & Zhang, Y. Role of Jhdm2a in regulating metabolic gene expression and obesity resistance. Nature 458, 757–761 (2009).
Inagaki, T. et al. Obesity and metabolic syndrome in histone demethylase JHDM2a-deficient mice. Genes Cells 14, 991–1001 (2009).
Mikkelsen, T. S. et al. Comparative epigenomic analysis of murine and human adipogenesis. Cell 143, 156–169 (2010).
Hotamisligil, G. S., Arner, P., Caro, J. F., Atkinson, R. L. & Spiegelman, B. M. Increased adipose tissue expression of tumor necrosis factor-α in human obesity and insulin resistance. J. Clin. Invest. 95, 2409–2415 (1995).
Uysal, K. T., Wiesbrock, S. M., Marino, M. W. & Hotamisligil, G. S. Protection from obesity-induced insulin resistance in mice lacking TNF-α function. Nature 389, 610–614 (1997).
Kusunoki, M., Cooney, G. J., Hara, T. & Storlien, L. H. Amelioration of high-fat feeding-induced insulin resistance in skeletal muscle with the antiglucocorticoid RU486. Diabetes 44, 718–720 (1995).
Kiortsis, D. N., Mavridis, A. K., Vasakos, S., Nikas, S. N. & Drosos, A. A. Effects of infliximab treatment on insulin resistance in patients with rheumatoid arthritis and ankylosing spondylitis. Ann. Rheum. Dis. 64, 765–766 (2005).
Defronzo, R. A. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 58, 773–795 (2009).
Lo, K. A. et al. in vitro insulin-resistance models and their physiological relevance to in vivo diet-induced adipose insulin resistance. Cell Rep. 5, 259–270 (2013).
Xing, H. et al. TNF α-mediated inhibition and reversal of adipocyte differentiation is accompanied by suppressed expression of PPARγ without effects on Pref-1 expression. Endocrinology 138, 2776–2783 (1997).
Nadler, S. T. et al. The expression of adipogenic genes is decreased in obesity and diabetes mellitus. Proc. Natl Acad. Sci. USA 97, 11371–11376 (2000).
Ernst, J. & Kellis, M. Discovery and characterization of chromatin states for systematic annotation of the human genome. Nat. Biotechnol. 28, 817–825 (2010).
Ernst, J. et al. Mapping and analysis of chromatin state dynamics in nine human cell types. Nature 473, 43–49 (2011).
Hoffman, M. M. et al. Integrative annotation of chromatin elements from ENCODE data. Nucleic Acids Res. 41, 827–841 (2013).
Creyghton, M. P. et al. Histone H3K27ac separates active from poised enhancers and predicts developmental state. Proc. Natl Acad. Sci. USA 107, 21931–21936 (2010).
Gelmann, E. P. Molecular biology of the androgen receptor. J. Clin. Oncol. 20, 3001–3015 (2002).
Htun, H., Barsony, J., Renyi, I., Gould, D. L. & Hager, G. L. Visualization of glucocorticoid receptor translocation and intranuclear organization in living cells with a green fluorescent protein chimera. Proc. Natl Acad. Sci. USA 93, 4845–4850 (1996).
Tomlinson, J. W. et al. 11β-hydroxysteroid dehydrogenase type 1: a tissue-specific regulator of glucocorticoid response. Endocr. Rev. 25, 831–866 (2004).
Yu, C. Y. et al. Genome-wide analysis of glucocorticoid receptor binding regions in adipocytes reveal gene network involved in triglyceride homeostasis. PLoS ONE 5, e15188 (2010).
Zeggini, E. et al. Meta-analysis of genome-wide association data and large-scale replication identifies additional susceptibility loci for type 2 diabetes. Nat. Genet. 40, 638–645 (2008).
de Alvaro, C., Teruel, T., Hernandez, R. & Lorenzo, M. Tumor necrosis factor α produces insulin resistance in skeletal muscle by activation of inhibitor κB kinase in a p38 MAPK-dependent manner. J. Biol. Chem. 279, 17070–17078 (2004).
del Aguila, L. F., Claffey, K. P. & Kirwan, J. P. TNF-α impairs insulin signaling and insulin stimulation of glucose uptake in C2C12 muscle cells. Am. J. Physiol. 276, E849–E855 (1999).
Hernandez, R., Teruel, T., de Alvaro, C. & Lorenzo, M. Rosiglitazone ameliorates insulin resistance in brown adipocytes of Wistar rats by impairing TNF-α induction of p38 and p42/p44 mitogen-activated protein kinases. Diabetologia 47, 1615–1624 (2004).
Hirosumi, J. et al. A central role for JNK in obesity and insulin resistance. Nature 420, 333–336 (2002).
Nohara, A., Okada, S., Ohshima, K., Pessin, J. E. & Mori, M. Cyclin-dependent kinase-5 is a key molecule in tumor necrosis factor-α-induced insulin resistance. J. Biol. Chem. 286, 33457–33465 (2011).
Yan, Q. W. et al. The adipokine lipocalin 2 is regulated by obesity and promotes insulin resistance. Diabetes 56, 2533–2540 (2007).
Jun, L. S., Siddall, C. P. & Rosen, E. D. A minor role for lipocalin 2 in high-fat diet-induced glucose intolerance. Am. J. Physiol. Endocrinol. Metab. 301, E825–E835 (2011).
Law, I. K. et al. Lipocalin-2 deficiency attenuates insulin resistance associated with aging and obesity. Diabetes 59, 872–882 (2010).
Olefsky, J. M. & Glass, C. K. Macrophages, inflammation, and insulin resistance. Annu. Rev. Physiol. 72, 219–246 (2010).
Kuo, T. et al. Genome-wide analysis of glucocorticoid receptor-binding sites in myotubes identifies gene networks modulating insulin signaling. Proc. Natl Acad. Sci. USA 109, 11160–11165 (2012).
Fleseriu, M. et al. Mifepristone, a glucocorticoid receptor antagonist, produces clinical and metabolic benefits in patients with Cushing’s syndrome. J. Clin. Endocrinol. Metab. 97, 2039–2049 (2012).
Lannan, E. A., Galliher-Beckley, A. J., Scoltock, A. B. & Cidlowski, J. A. Proinflammatory actions of glucocorticoids: glucocorticoids and TNFα coregulate gene expression in vitro and in vivo. Endocrinology 153, 3701–3712 (2012).
Uhlenhaut, N. H. et al. Insights into negative regulation by the glucocorticoid receptor from genome-wide profiling of inflammatory cistromes. Mol. Cell 49, 158–171 (2013).
De Bosscher, K., Vanden Berghe, W. & Haegeman, G. The interplay between the glucocorticoid receptor and nuclear factor-κB or activator protein-1: molecular mechanisms for gene repression. Endocr. Rev. 24, 488–522 (2003).
Arkan, M. C. et al. IKK-β links inflammation to obesity-induced insulin resistance. Nat. Med. 11, 191–198 (2005).
Cai, D. et al. Local and systemic insulin resistance resulting from hepatic activation of IKK-β and NF-κB. Nat. Med. 11, 183–190 (2005).
Yuan, M. et al. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkβ. Science 293, 1673–1677 (2001).
Zhang, J., Gao, Z., Yin, J., Quon, M. J. & Ye, J. S6K directly phosphorylates IRS-1 on Ser-270 to promote insulin resistance in response to TNF-α signaling through IKK2. J. Biol. Chem. 283, 35375–35382 (2008).
Tang, T. et al. Uncoupling of inflammation and insulin resistance by NF-κB in transgenic mice through elevated energy expenditure. J. Biol. Chem. 285, 4637–4644 (2010).
Gao, Z. et al. Inactivation of NF-κB p50 leads to insulin sensitization in liver through post-translational inhibition of p70S6K. J. Biol. Chem. 284, 18368–18376 (2009).
Sutter-Dub, M. T. Carbohydrate metabolism of female rat adipocytes: effects and mechanisms of action of progesterone. Diabete Metab. 12, 329–336 (1986).
Kelly, D. M. & Jones, T. H. Testosterone: a metabolic hormone in health and disease. J. Endocrinol. 217, R25–R45 (2013).
Mezza, T. et al. Vitamin D deficiency: a new risk factor for type 2 diabetes? Ann. Nutr. Metab. 61, 337–348 (2012).
Sung, C. C., Liao, M. T., Lu, K. C. & Wu, C. C. Role of vitamin D in insulin resistance. J. Biomed. Biotechnol. 2012, 634195 (2012).
Chen, J. D. & Evans, R. M. A transcriptional co-repressor that interacts with nuclear hormone receptors. Nature 377, 454–457 (1995).
Yu, C. et al. The nuclear receptor corepressors NCoR and SMRT decrease peroxisome proliferator-activated receptor γ transcriptional activity and repress 3T3-L1 adipogenesis. J. Biol. Chem. 280, 13600–13605 (2005).
Lin, H. M., Zhao, L. & Cheng, S. Y. Cyclin D1 is a ligand-independent co-repressor for thyroid hormone receptors. J. Biol. Chem. 277, 28733–28741 (2002).
Heikkinen, S. et al. Nuclear hormone 1α,25-dihydroxyvitamin D3 elicits a genome-wide shift in the locations of VDR chromatin occupancy. Nucleic Acids Res. 39, 9181–9193 (2011).
Ramagopalan, S. V. et al. A ChIP-seq defined genome-wide map of vitamin D receptor binding: associations with disease and evolution. Genome Res. 20, 1352–1360 (2010).
Wong, K. E. et al. Targeted expression of human vitamin D receptor in adipocytes decreases energy expenditure and induces obesity in mice. J. Biol. Chem. 286, 33804–33810 (2011).
Kang, S. et al. Regulation of early adipose commitment by Zfp521. PLoS Biol. 10, e1001433 (2012).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25, 402–408 (2001).
Hubbell, E., Liu, W. M. & Mei, R. Robust estimators for expression analysis. Bioinformatics 18, 1585–1592 (2002).
Tusher, V. G., Tibshirani, R. & Chu, G. Significance analysis of microarrays applied to the ionizing radiation response. Proc. Natl Acad. Sci. USA 98, 5116–5121 (2001).
Dennis, G. Jr et al. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 4, P3 (2003).
Mikkelsen, T. S. et al. Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature 448, 553–560 (2007).
Adli, M., Zhu, J. & Bernstein, B. E. Genome-wide chromatin maps derived from limited numbers of hematopoietic progenitors. Nat. Methods 7, 615–618 (2010).
Shechter, D., Dormann, H. L., Allis, C. D. & Hake, S. B. Extraction, purification and analysis of histones. Nat. Protoc. 2, 1445–1457 (2007).
Rappsilber, J., Ishihama, Y. & Mann, M. Stop and go extraction tips for matrix-assisted laser desorption/ionization, nanoelectrospray, and LC/MS sample pretreatment in proteomics. Anal. Chem. 75, 663–670 (2003).
DiMaggio, P. A. Jr, Young, N. L., Baliban, R. C., Garcia, B. A. & Floudas, C. A. A mixed integer linear optimization framework for the identification and quantification of targeted post-translational modifications of highly modified proteins using multiplexed electron transfer dissociation tandem mass spectrometry. Mol. Cell. Proteomics 8, 2527–2543 (2009).
Wu, Y., Dimaggio, P. A., Perlman, D. H. Jr, Zakian, V. A. & Garcia, B. A. Novel phosphorylation sites in the S. cerevisiae Cdc13 protein reveal new targets for telomere length regulation. J. Proteome Res. 12, 316–327 (2013).
Li, H., Ruan, J. & Durbin, R. Mapping short DNA sequencing reads and calling variants using mapping quality scores. Genome Res. 18, 1851–1858 (2008).
Quinlan, A. R. & Hall, I. M. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26, 841–842 (2010).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010).
Ernst, J. & Kellis, M. ChromHMM: automating chromatin-state discovery and characterization. Nat. Methods 9, 215–216 (2012).
Matys, V. et al. TRANSFAC and its module TRANSCompel: transcriptional gene regulation in eukaryotes. Nucleic Acids Res. 34, D108–D110 (2006).
Kel, A. E. et al. MATCH: a tool for searching transcription factor binding sites in DNA sequences. Nucleic Acids Res. 31, 3576–3579 (2003).
Bailey, T. L. et al. MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res. 37, W202–W208 (2009).
Acknowledgements
We would like to thank M. Herman (Beth Israel Deaconess Medical Center, USA) for providing adipose RNA from obese mice. We thank members of the Rosen laboratory for helpful discussions, and E. Merkel for technical assistance. The V729I and D641V GR mutant alleles were from G. Chrousos (Athens University Medical School, Greece), and the N768 allele was a gift from K. Yamamoto (University of California, San Francisco, USA). J-C. Wang (University of California, Berkeley, USA) provided the anti-GR antibody used for ChIP-PCR and ChIP-Seq, and he and I. Rogatsky were generous with their time and advice. This work was supported by NIH Roadmap grant R01 ES017690, R01085171 and an American Diabetes Association Career Development Award to E.D.R., NIH Innovator grant DP2OD007447 to B.A.G., and by American Heart Association Postdoctoral Awards to S.K. and L.T.T.
Author information
Authors and Affiliations
Contributions
S.K., L.T.T. and E.D.R. designed the study. Experimental work was carried out by S.K., with help from M.J.G. and S.X.; ChIP-Seq was performed by S.K. and L.T.T. with assistance from R.I., H.J.W. and C.B.E. Computational data analysis was performed by L.T.T., Y.Z. and T.S.M. A.E. and B.A.G. performed histone mass spectrometry. S.K., L.T.T. and E.D.R. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 The Dex and TNF model system.
(a) Cartoon depicting the Dex-TNF system. 3T3-L1 adipocytes are differentiated fully for 8 days, and then treated with Dex or TNF for a variable amount of time (6 days maximum) before glucose uptake assay or harvest for RNA or ChIP. Of note, drug treatments were staggered so that cells were always harvested 14 days after confluence was reached, thus ensuring that results do not reflect different duration of culture. (b) Oil red O staining of cells treated with Dex or TNF for 6 days. (c) Expression of differentiation markers in cells treated with Dex or TNF for 6 days (data represent mean of n = 3 dishes, data from 1 additional independent experiment shown in Source Data Table). (d) Heat map of correlation coefficients observed after comparing transcriptional profiles from Dex- and TNF-treated cells to those obtained at different stages of adipogenesis (from14). (e) Western blot of PPARγ levels in cells from c. (f) quantification of e (data show the result from one experiment). (g) Heat map of treatment-sensitive gene expression changes from Dex- and TNF-treated cells. The colour bar at right denotes 9 distinct clusters representing different patterns of expression in response to Dex and TNF. (h) Venn diagram depicting the number of down-regulated genes in response to Dex and TNF treatment. These numbers represent a ‘unity’ set in which all time points were combined.
Supplementary Figure 2 Comparison of transcriptional events due to Dex and TNF versus diet-induced obesity.
(a–c) Genes concordantly up-regulated (red) and down-regulated (green) by Dex, TNF, or both (overlapping) are mapped onto a plot of genes altered in two murine models of obesity, high-fat fed C57Bl/6 and BTBR mice (grey). Obesity data are from22. X and Y axes reflect log2 fold change (HFD versus Chow). (d) Box-and-whiskers plot of data from a–c, comparing genes up- and down-regulated by Dex, TNF, or both to obese C57Bl/6 mice (n = number of genes in each group (invariant = 160, DexUP = 357, TNF_UP = 512, Overlap_UP = 110, Dex_Down = 358, TNF_Down = 339, Overlap_ DOWN = 120), whiskers represent 1 SD, box edges are 1st and 3rd quartiles, Student’s t-test, p < 0.05). (e) Box-and-whiskers plot of data from a–c, comparing genes up- and down-regulated by Dex, TNF, or both to obese BTBR mice (n values as in d, Student’s t-test, p < 0.05). (f) Fold change (log2 scale) of induced or repressed concordantly by Dex and TNF in the white adipose tissue of two strains of obese versus lean mice (n values as in d, p < 0.05 by Student’s t-test, mean ± SD). (g) Heat map of correlation coefficients comparing gene expression changes from specific Dex and TNF time points to the C57Bl/6 and BTBR obesity models.
Supplementary Figure 3 Epigenomic analysis of cellular insulin resistance
(a) Trypsinized histone fragments from Dex- and TNF-treated cells were subjected to mass spectrometry. Depicted is a heat map displaying changes in histone 3 marks (n = 2). (b) ChIP-Seq was performed using antibodies directed against the indicated histone modifications at different time points after Dex and TNF treatment. Shown are the total number of called peaks for each modification and time point. (c) Heat maps for model parameters from ChromHMM model learned from x-axis labelled ChIP-seq data sets across control and all Dex and TNF time points. The columns indicate the relative percentage of the genome represented by each chromatin state. WCE = whole-cell extract. (d) Heat maps for enrichment of each state for various annotated functional genomic elements e. Heat maps for enrichment of each state as a function of distance from TSS. (f) Numbers of promoters and enhancers during adipocyte differentiation (compared to Day 7 adipocytes), or at each time point after Dex and TNF treatment (compared to untreated) are indicated. The percentages of nonoverlapping promoters or enhancers that change with differentiation or drug treatment are shown at right.
Supplementary Figure 4 Genes coordinately up-regulated by Dex and TNF lie near up-regulated H3K27ac peaks.
(a) Venn diagram depicting genes induced by Dex and TNF. Of the 271 genes coordinately induced by both agents, 147 were flanked by at least one up-regulated H3K27ac peak within ±200 kb of the TSS. These 147 genes could be classified as associated with a ‘Dex-only’ peak (n = 22), a ‘TNF-only peak’ (n = 80), or both a Dex and a TNF-induced peak (n = 147). This last group could be further subcategorized into ‘Dex-TNF nonoverlapping’ peaks (n = 99) and ‘Dex-TNF overlapping’ peaks (n = 48). (b) Tracks from an up-regulated gene (Cebpd) showing a ‘Dex-only’ H3K27ac peak. (c) Tracks from an up-regulated gene (Igfbp3) showing a ‘TNF-only’ H3K27ac peak. (d) Tracks from an up-regulated gene (Ifngr1) showing a ‘Dex-TNF-nonoverlapping’ H3K27ac peak. (e) Tracks from an up-regulated gene (Fam46b) showing a ‘Dex-TNF-overlapping’ H3K27ac peak.
Supplementary Figure 5 TNF induces insulin resistance in part via ligand-independent activation of the GR.
(a) Expression of Nr3c1 (GR) in cells 6 days after transduction with lentiviruses carrying shRNAs directed against GR or a scrambled control (shScr) (data represent mean of n = 3 dishes, data from 1 additional independent experiment shown in Source Data Table). (b) Expression of Rela (p65) in cells 6 days after transduction with lentiviruses carrying shRNAs directed against p65 or a scrambled control (shScr) (data represent mean of n = 3 dishes, data from 1 additional independent experiment shown in Source Data Table). (c) Several shRNAs against p65 (vs. scrambled shRNA: shScr) were delivered to mature adipocytes via lentivirus, and cells were then treated with Dex or TNF and assessed for insulin-stimulated glucose uptake. Shown is the percent of insulin-stimulated glucose uptake rescued by p65 knockdown (data represent mean of n = 6 dishes, data from 2 additional independent experiments shown in Source Data Table); ND = not determined because of cell death). (d) Expression of Hsd11b1 was assessed in mature 3T3-L1 adipocytes by Q-PCR after treatment with Dex and TNF for the indicated period of time (data represent mean of n = 3 dishes, data from 1 additional independent experiment shown in Source Data Table). (e) Carbenoxolone at the indicated dose was treated to fully differentiated adipocytes with or without Dex or TNF for 6 days and then assessed for insulin-stimulated glucose uptake (data represent mean of n = 6 dishes, data from 1 additional independent experiment shown in Source Data Table). (f) The effect of carbenoxolone (70 μM) was tested on glucocorticoid-dependent gene expression in mature 3T3-L1 adipocytes. Cortisol or cortisone (1 uM each) were used to induce expression of known targets Sgk1 and Dusp1; carbenoxolone blocks the effect of cortisone, which must be activated by 11-β-HSD1, but not cortisol, which does not require activation, (data represent mean of n = 3 dishes, data from 1 additional independent experiment shown in Source Data Table).
Supplementary Figure 6 TNF and Dex induce binding of GR to specific loci.
(a) Genomic distribution of significantly enriched GR peaks. (b) Mean mammalian conservation scores of GR peak subsets centered around GR peak centers. The gray dotted line represents mean conservation score across entire genome. (c) Heat map showing SQRT –log2 (p-value) comparing the fold change in expression for the nearest gene from peak sets at the indicated condition/time point to a random set of H3K27ac peaks (fold changes are provided in Fig. 5c). Non-significant p-values after multiple testing hypothesis correction are labeled N.S.
Supplementary Figure 7 VDR binds to ‘Dex-TNF-overlapping’ H3K27ac peaks.
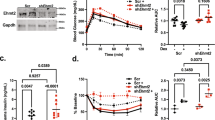
(a) Tracks from an up-regulated gene (Lcn2) showing a ‘Dex-TNF-overlapping’ H3K27ac peak. (b) ChIP-PCR results from the ‘Dex-TNF-overlapping’ peak depicted in a (data represent mean of n = 3 dishes, data from 2 additional independent experiments shown in Source Data Table). (c) Tracks from an up-regulated gene cluster (Tmem176a/b) showing a ‘Dex-TNF-overlapping’ H3K27ac peak. (d) ChIP-PCR results from the ‘Dex-TNF-overlapping’ peak depicted in c (data represent mean of n = 3 dishes, data from 1 additional independent experiment shown in Source Data Table). (e) ChIP-PCR of VDR and GR from a nonspecific region near Lcn2 (data represent mean of n = 3 dishes, data from 1 additional independent experiment shown in Source Data Table). (f) ChIP-PCR of VDR and GR from a nonspecific region near Tmem176a/b (data represent mean of n = 3 dishes, data from 1 additional independent experiment shown in Source Data Table). (g) RXR ChIP-PCR results in 3T3-L1 adipocytes from the ‘Dex-TNF-overlapping’ peaks containing VDR motifs depicted in Figs. S5g, S10a, and S10c near Colq, Tmem176a, and Lcn2 loci (data represent mean of n = 3 dishes, data from 2 additional independent experiments shown in Source Data Table), using anti-RXR or IgG. (h) Expression of flag-tagged VDR in cells 4 days after transduction with lentivirus. (i) Expression of Vdr in cells 6 days after transduction with lentiviruses carrying shRNAs directed against VDR or a scrambled control (shScr) (data represent mean of n = 3 dishes, data from 1 additional independent experiment shown in Source Data Table). (j) Fully differentiated adipocytes were pre-treated with vehicle, NAC (1 mM) or MnTABP (100 nM) for 2 days and then treated with Dex or TNF for an additional 6 days and assessed for insulin-stimulated glucose uptake (data represent mean of n = 6 dishes, data from 1 additional independent experiment shown in Source Data Table). (k) After overexpressing VDR or control vector, cells were treated with NAC (1 mM), MnTBAP (100 nM), or vehicle and assessed for insulin-stimulated glucose uptake (data represent mean of n = 6 dishes, data from 1 additional independent experiment shown in Source Data Table). (l) Vdr mRNA levels were measured in samples collected from the experiment in j (data represent mean of n = 3 dishes, data from 1 additional independent experiment shown in Source Data Table).
Supplementary Figure 8 Assessment of GR and VDR target genes.
(a) Q-PCR validation of coordinately up-regulated genes. Cells were treated with Dex (blue) or TNF (red) for the indicated lengths of time before RNA harvest (data represent mean of n = 3 dishes, data from 1 additional independent experiment shown in Source Data Table). (b) Expression of up-regulated target genes in cells transduced with lentivirus carrying shRNA directed against VDR or GR (vs. scrambled control: shScr) and then treated with Dex (D) or TNF (T) for 6 days (data represent mean of n = 3 dishes, data from 1 additional independent experiment shown in Source Data Table). (c) Expression of coordinately up-regulated genes was measured in white adipose tissue samples from chow and high fat-fed C57Bl/6 mice (n = 6 mice P < 0.05, Student’s t-test, mean ± SEM). (d) Expression of coordinately up-regulated genes was measured in white adipose tissue samples from ob/+ and ob/ob mice, treated with vehicle or rosiglitazone for 6 weeks (n = 7 for ob/+, n = 8 for ob/+ plus Rosi, n = 7 for ob/ob with and without Rosi, P < 0.05, Student’s t-test, mean ± SEM).
Supplementary Figure 9 Uncropped blots used in this manuscript.
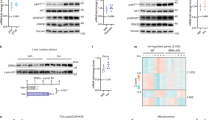
(a–c) Scanned images for GR fractionation assays shown in main Fig. 3g (a: Flag-GR, b: TBP, and c: GAPDH). (d,e) Scanned images for total GR protein from whole cell lysates shown in main Fig. 3g (c: Flag-GR, d: GAPDH). (f,g) Scanned images for PPARγ levels during the time course of Dex and TNF treatment in Supplementary Fig. 1e (f: PPARγ, g: Histone3), (h,i) Scanned images for the measurement of VDR overexpression in Supplementary Fig. 7h (h: Flag-VDR, i: GAPDH).
Supplementary information
Supplementary Information
Supplementary Information (PDF 3232 kb)
Supplementary Table 1
Supplementary Information (XLS 42 kb)
Supplementary Table 2
Supplementary Information (XLS 1328 kb)
Supplementary Table 3
Supplementary Information (XLS 47 kb)
Supplementary Table 4
Supplementary Information (XLS 197 kb)
Supplementary Table 5
Supplementary Information (XLS 53 kb)
Supplementary Table 6
Supplementary Information (XLS 38 kb)
Supplementary Table 7
Supplementary Information (XLSX 354 kb)
Rights and permissions
About this article
Cite this article
Kang, S., Tsai, L., Zhou, Y. et al. Identification of nuclear hormone receptor pathways causing insulin resistance by transcriptional and epigenomic analysis. Nat Cell Biol 17, 44–56 (2015). https://doi.org/10.1038/ncb3080
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb3080
This article is cited by
-
Insulin resistance rewires the metabolic gene program and glucose utilization in human white adipocytes
International Journal of Obesity (2022)
-
Obesity-associated inflammation triggers an autophagy–lysosomal response in adipocytes and causes degradation of perilipin 1
Cell Death & Disease (2019)
-
Epigenetics and Type 2 Diabetes Risk
Current Diabetes Reports (2019)
-
Biochemical and cellular properties of insulin receptor signalling
Nature Reviews Molecular Cell Biology (2018)
-
HO-1 inhibits preadipocyte proliferation and differentiation at the onset of obesity via ROS dependent activation of Akt2
Scientific Reports (2017)