Abstract
Gastrulation leads to three germ layers—ectoderm, mesoderm and endoderm—that are separated by two basement membranes. In the mouse embryo, the emergent gut endoderm results from the widespread intercalation of cells of two distinct origins: pluripotent epiblast-derived definitive endoderm (DE) and extra-embryonic visceral endoderm (VE). Here we image the trajectory of prospective DE cells before intercalating into the VE epithelium. We show that the transcription factor SOX17, which is activated in prospective DE cells before intercalation, is necessary for gut endoderm morphogenesis and the assembly of the basement membrane that separates gut endoderm from mesoderm. Our results mechanistically link gut endoderm morphogenesis and germ layer segregation, two central and conserved features of gastrulation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Nowotschin, S. & Hadjantonakis, A. K. Cellular dynamics in the early mouse embryo: from axis formation to gastrulation. Curr. Opin. Genet. Dev. 20, 420–427 (2010).
Arnold, S. J. & Robertson, E. J. Making a commitment: cell lineage allocation and axis patterning in the early mouse embryo. Nat. Rev. Mol. Cell Biol. 10, 91–103 (2009).
Rivera-Perez, J. A. & Hadjantonakis, A. K. The Dynamics of Morphogenesis in the Early Mouse Embryo (Cold Spring Harbor Perspectives, 2014).
Zorn, A. M. & Wells, J. M. Vertebrate endoderm development and organ formation. Annu. Rev. Cell Dev. Biol. 25, 221–251 (2009).
Viotti, M., Nowotschin, S. & Hadjantonakis, A. K. Afp::mCherry, a red fluorescent transgenic reporter of the mouse visceral endoderm. Genesis 49, 124–133 (2011).
Kwon, G. S., Viotti, M. & Hadjantonakis, A. K. The endoderm of the mouse embryo arises by dynamic widespread intercalation of embryonic and extraembryonic lineages. Dev. Cell 15, 509–520 (2008).
Viotti, M., Niu, L., Shi, S. H. & Hadjantonakis, A. K. Role of the gut endoderm in relaying left-right patterning in mice. PLoS Biol. 10, e1001276 (2012).
Mesnard, D., Guzman-Ayala, M. & Constam, D. B. Nodal specifies embryonic visceral endoderm and sustains pluripotent cells in the epiblast before overt axial patterning. Development 133, 2497–2505 (2006).
Kwon, G. S. et al. Tg(Afp-GFP) expression marks primitive and definitive endoderm lineages during mouse development. Dev. Dyn. 235, 2549–2558 (2006).
Yamaguchi, T. P., Harpal, K., Henkemeyer, M. & Rossant, J. fgfr-1 is required for embryonic growth and mesodermal patterning during mouse gastrulation. Genes Dev. 8, 3032–3044 (1994).
Ciruna, B. & Rossant, J. FGF signaling regulates mesoderm cell fate specification and morphogenetic movement at the primitive streak. Dev. Cell 1, 37–49 (2001).
Sun, X., Meyers, E. N., Lewandoski, M. & Martin, G. R. Targeted disruption of Fgf8 causes failure of cell migration in the gastrulating mouse embryo. Genes Dev. 13, 1834–1846 (1999).
Nowotschin, S. et al. The T-box transcription factor Eomesodermin is essential for AVE induction in the mouse embryo. Genes Dev. 27, 997–1002 (2013).
Teo, A. K. et al. Pluripotency factors regulate definitive endoderm specification through eomesodermin. Genes Dev. 25, 238–250 (2011).
Arnold, S. J., Hofmann, U. K., Bikoff, E. K. & Robertson, E. J. Pivotal roles for eomesodermin during axis formation, epithelium-to-mesenchyme transition and endoderm specification in the mouse. Dev. Suppl. 135, 501–511 (2008).
Kanai-Azuma, M. et al. Depletion of definitive gut endoderm in Sox17-null mutant mice. Dev. Suppl. 129, 2367–2379 (2002).
Seguin, C. A., Draper, J. S., Nagy, A. & Rossant, J. Establishment of endoderm progenitors by SOX transcription factor expression in human embryonic stem cells. Cell Stem Cell 3, 182–195 (2008).
Stainier, D. Y. A glimpse into the molecular entrails of endoderm formation. Genes Dev. 16, 893–907 (2002).
Kim, I., Saunders, T. L. & Morrison, S. J. Sox17 dependence distinguishes the transcriptional regulation of fetal from adult hematopoietic stem cells. Cell 130, 470–483 (2007).
Niakan, K. K. et al. Sox17 promotes differentiation in mouse embryonic stem cells by directly regulating extraembryonic gene expression and indirectly antagonizing self-renewal. Genes Dev. 24, 312–326 (2010).
Baum, B. & Georgiou, M. Dynamics of adherens junctions in epithelial establishment, maintenance, and remodeling. J. Cell Biol. 192, 907–917 (2011).
Roh, M. H. & Margolis, B. Composition and function of PDZ protein complexes during cell polarization. Am. J. Physiol. Renal Physiol. 285, F377–F387 (2003).
Humbert, P. O., Dow, L. E. & Russell, S. M. The Scribble and Par complexes in polarity and migration: friends or foes? Trends Cell Biol. 16, 622–630 (2006).
Vakaet, L. Some new data concerning the formation of the definitive endoblast in the chick embryo. J. Embryol. Exp. Morphol. 10, 38–57 (1962).
Azar, Y. & Eyal-Giladi, H. The retention of primary hypoblastic cells underneath the developing primitive streak allows for their prolonged inductive influence. J. Embryol. Exp. Morphol. 77, 143–151 (1983).
Kimura, W., Yasugi, S., Stern, C. D. & Fukuda, K. Fate and plasticity of the endoderm in the early chick embryo. Dev. Biol. 289, 283–295 (2006).
Bertocchini, F. & Stern, C. D. A differential screen for genes expressed in the extraembryonic endodermal layer of pre-primitive streak stage chick embryos reveals expression of Apolipoprotein A1 in hypoblast, endoblast and endoderm. Gene Expr. Patterns 8, 477–480 (2008).
Ang, S. L. & Rossant, J. HNF-3 beta is essential for node and notochord formation in mouse development. Cell 78, 561–574 (1994).
Weinstein, D. C. et al. The winged-helix transcription factor HNF-3 beta is required for notochord development in the mouse embryo. Cell 78, 575–588 (1994).
McKnight, K. D., Hou, J. & Hoodless, P. A. Foxh1 and Foxa2 are not required for formation of the midgut and hindgut definitive endoderm. Dev. Biol. 337, 471–481 (2010).
Meyers, E. N., Lewandoski, M. & Martin, G. R. An Fgf8 mutant allelic series generated by Cre- and Flp-mediated recombination. Nat. Genet. 18, 136–141 (1998).
Mao, C. A. et al. Eomesodermin, a target gene of Pou4f2, is required for retinal ganglion cell and optic nerve development in the mouse. Development 135, 271–280 (2008).
Hayashi, S., Lewis, P., Pevny, L. & McMahon, A. P. Efficient gene modulation in mouse epiblast using a Sox2Cre transgenic mouse strain. Mech. Dev. 119 (Suppl. 1), S97–S101 (2002).
Downs, K. M. & Davies, T. Staging of gastrulating mouse embryos by morphological landmarks in the dissecting microscope. Development 118, 1255–1266 (1993).
Jones, E. A. V., Hadjantonakis, A. K. & Dickinson, M. E. in Imaging in Neuroscience and Development (eds Yuste, R. & Konnerth, A.) (Cold Spring Harbor Laboratory Press, 2005).
Khoo, P. L., Franklin, V. J. & Tam, P. P. Fate-mapping technique: targeted whole-embryo electroporation of DNA constructs into the germ layers of mouse embryos 7–7.5 days post-coitum. CSH Protoc. 2007 (2007)10.1101/pbd.prot4893
Long, J. Z., Lackan, C. S. & Hadjantonakis, A. K. Genetic and spectrally distinct in vivo imaging: embryonic stem cells and mice with widespread expression of a monomeric red fluorescent protein. BMC Biotechnol. 5, 20 (2005).
Nowotschin, S., Eakin, G. S. & Hadjantonakis, A. K. Dual transgene strategy for live visualization of chromatin and plasma membrane dynamics in murine embryonic stem cells and embryonic tissues. Genesis 47, 330–336 (2009).
Nowotschin, S., Ferrer-Vaquer, A. & Hadjantonakis, A. K. Imaging mouse development with confocal time-lapse microscopy. Methods Enzymol. 476, 351–377 (2010).
Acknowledgements
We thank G. Kwon for implementing and optimizing the embryo electroporation technique and live imaging; M. Pulina for assistance with analysis of FGF signalling mutants; R. Turnbull for assistance with embryo cryosectioning; V. Seshan of the MSKCC Biostatistics Core Facility for advice on fluorescence intensity quantifications; J. Darnell, G. Martin, S. Morrison, C-a. Mao and J. Rossant for mouse lines; K. Anderson, M. Baylies and members of our laboratory for discussions and comments on the manuscript. This work was supported by the NIH (RO1-HD052115 and RO1-DK084391). M.V. was supported by a Frank J. Lappin Horsfall fellowship for part of this work S.N. was supported by a Muscular Dystrophy Association Development Grant (186552).
Author information
Authors and Affiliations
Contributions
A-K.H. conceived the project. M.V., S.N. and A-K.H. designed the experiments and interpreted results. M.V. and S.N. carried out the experiments. M.V. and A-K.H. wrote the manuscript with input from S.N.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 2 Gastrulation mutants fail to disperse the emVE.
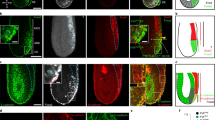
(a and a′) Whole mount and transverse section of Fgfr1 mutant expressing the Afp::GFP VE-reporter at PS stage, showing a solid emVE layer on the embryo’s surface. (b and b′) Whole mount and transverse section of Fgfr1 mutant expressing the Afp::GFP VE-reporter at OB stage, showing no mesodermal migration and no emVE dispersal. (c and c′) Whole mount and transverse section of epiblast-ablated Eomes mutant expressing the Afp::GFP VE-reporter at OB stage, showing thick epiblast layer, no wings of mesoderm migration, and no emVE dispersal. ps, primitive streak; A, anterior; D, distal; L, left; P, posterior; Pr, proximal; R, right; PS, pre-streak; OB, no bud. Scale bars = 100 μm.
Supplementary Figure 3 Expression of SOX17 and FOXA2 in VE and DE cells.
(a-f′) Immuno-fluorescence for SOX17 in Afp::GFP VE-reporter embryos in progressive stages of gastrulation. (g) Quantitation of fluorescent intensity show high levels of SOX17 in egressing DE cells, and subsequent equalization of expression levels between DE cells and emVE cells. (h–m′) Immuno-fluorescence for FOXA2 in Afp::GFP VE-reporter embryos in progressive stages of gastrulation. (n) Quantitation of fluorescent intensity show high levels of FOXA2 in egressing DE cells, and subsequent equalization of expression levels between DE cells and emVE cells. (o–v) Double immuno-fluorescence for FOXA2 and SOX17 in a mid-emVE dispersal Afp::GFP wild-type embryo. DE cells display high levels of SOX17 as well as FOXA2 (white arrowheads). Both SOX17 and FOXA2 are expressed at low levels in GFP-positive emVE cells (orange arrowheads). (s′–v′) Sox17 mutants show absence of SOX17 but presence of FOXA2 in the cells of the mesodermal wings bordering the emVE. FOXA2 is also present at lower levels in GFP-positive emVE cells. (w–x) Anterior views of Afp::GFP embryos at the EHF/LHF stage (E7.75) with immuno-fluorescence for SOX17 or FOXA2. Node and anterior midline are devoid of SOX17 and show strong localization of FOXA2. (y) Double immuno-fluorescence staining for SOX17 and FOXA2 in sequentially staged Afp::GFPTg/+ embryos. These data reveal low SOX17 expression in VE cells at pre-dispersal stages and upregulation once VE dispersal initiates. Strong SOX17 expression is present in egressing DE cells. Low levels of FOXA2 are detected in some epiblast cells overlying the region of the anterior primitive streak (white arrowheads) or in certain cells leaving the primitive streak. FOXA2 expression is high in egressing cells. Note, blue arrowhead marks FOXA2-positive cells during notochord formation. n, node; m, midline, ps, primitive streak; end, endoderm; epi, epiblast; mes, mesoderm; A, anterior; D, distal; L, left; P, posterior; Pr, proximal; R, right; PS, pre-streak; LS, late streak; OB, no bud; LB, late bud; EHF, early head-fold; LHF, late head-fold. Scale bars in a–x = 100 μm. Scale bars in y = 50 μm (whole mounts) and 20 μm (sections).
Supplementary Figure 4 N-CAD localizes between neighbouring cells that failed to egress in the absence of SOX17.
(a–c) Section through LB/EHF (E7.5) Sox17GFP/GFP embryo, showing N-CAD at the interface (blue arrowheads) of neighbouring non-egressed DE cells (blue asterisks). Pink asterisks mark emVE cells at the surface of the embryo. end, endoderm; epi, epiblast; mes, mesoderm; LB, late bud; EHF, early head-fold. Scale bars = 20 μm.
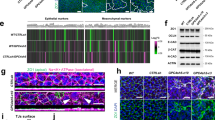
Supplementary Figure 5 BM expression and localization dynamics in wild-type and Sox17 mutant embryos.
(a) LAMA-1 immuno-fluorescence in Afp::GFP VE-reporter embryos. Note the single BM at early stages, and the progressive establishment of a second BM. Orange asterisks mark the anterior tips of the wings of mesoderm. (b) LAMA-1 localization in Fgf8 mutant Afp::GFP VE-reporter at the OB stage (E7.25) indicates a single BM. (c and d) High magnifications of FN-1 or LAMA-1 immuno-fluorescence in Afp::GFP embryos. Egressing DE cells (marked with pink asterisks) always display FN-1 and LAMA-1 basally, never at their apical interface with emVE cells. (e) Double immuno-fluorescence stains in Afp::GFP VE-reporter embryos show egressing DE cells with high SOX17 inserting between emVE cells, displaying FN-1 on their basal side. EmVE cells never display FN-1 at their interface with egressing cells. (f) Afp::GFP VE-reporter embryo stained for COLL-IV. In wild-types, egressing DE cells (orange asterisks) always display enriched COLL-IV basally. EmVE cells display cytoplasmic localization of COLL-IV. In Sox17 mutants, the interface between mesoderm and emVE displays faint COLL-IV fluorescent signal. Digital quantitation of fluorescent signal indicates three peaks for the COLL-IV signal in the wild-type. Digital quantitation of fluorescent signal in Sox17 mutants only displays one peak. (g) Afp::GFP VE-reporter embryos stained for LAMB-1. In wild-types, egressing DE cells (orange asterisks) always display enriched LAMB-1 basally. In Sox17 mutants, the interface between mesoderm and emVE only displays faint LAMB-1 fluorescent signal. Digital quantitation indicates two peaks for the wild-type LAMB-1 signal and only one peak in the Sox17 mutant. (h) In situ hybridization for Fn1 indicating transcription in the wings of mesoderm and overlying emVE. Potential DE cells showed intense staining, suggesting high levels of transcription (white arrowheads). (i) Fn1 in Sox17 mutant embryos is present in all cells of the mesodermal wings and overlying emVE. ps, primitive streak; emVE, embryonic visceral endoderm; end, endoderm; epi, epiblast; mes, mesoderm; A, anterior; D, distal; L, left; P, posterior; Pr, proximal; R, right; PS, pre-streak; LS, late streak; OB, no bud; LB, late bud; EHF, early head-fold. Scale bars = 100 μm in (a,b,f,g), 25 μm in (c–e).
Supplementary Figure 6 Analysis of E-CADHERIN localization during gut endoderm morphogenesis.
(a–e6) Immuno-fluorescence staining for E-CAD on Afp::GFPTg/+ embryos depicting changes of E-CAD localization from pre- through late stages of VE dispersal. White arrowheads depict E-CAD expression at interfaces between VE cells, pink arrowheads highlight expression at interfaces between VE and egressing cells during emVE dispersal, and blue arrowheads depict cell–cell junctions between egressing cells. White circles mark the junction between the extra-embryonic ectoderm and epiblast, namely the extra-embryonic/embryonic boundary, which coincides with the boundary of extra-embryonic (exVE) and embryonic visceral endoderm (emVE) only at LHF stages. ps, primitive streak; end, endoderm; epi, epiblast; mes, mesoderm; A, anterior; D, distal; L, left; P, posterior; Pr, proximal; R, right; PS, pre-streak; ES, early streak; OB, no bud; HF, head-fold; LHF, late head-fold. Scale bars = 50 μm (whole mounts) and 20 μm (sections).
Supplementary Figure 7 Analysis of ITGA5 localization during gut endoderm morphogenesis.
(a) Transcriptomic expression quantitation of integrins in E7.5 wild-type embryos. N = 3 (3 distal regions of wild-type embryos), graph depicts data points (circles) and mean with SD (horizontal lines). The complete data set is deposited in NCBI’s Gene Expression Omnibus (GEO), where it is publicly accessible under the code GSE33353. (b and c) Transverse section through Afp::GFP VE-reporter wild-type embryo at the OB stage (E7.25) immuno-fluorescently stained for ITGA5. (b′ and c′) Magnifications of boxed regions in (b) and (c), showing isotropic distribution of ITGA5 in inner cells of the mesodermal wings (yellow asterisks), and polarized enrichment in egressing cells (orange asterisks) at the cell membrane section facing the embryo cavity, their prospective basal side. ps, primitive streak; end, endoderm; epi, epiblast; mes, mesoderm; A, anterior; L, left; P, posterior; R, right; OB, no bud. Scale bars = 20μm.
Supplementary Figure 8 Method used for immuno-fluorescent digital signal intensity quantitation.
(a) Example of image used for quantitation of immuno-fluorescent signal. Section through Afp::GFP VE-reporter transgenic embryo stained for E-CADHERIN and ACTIN at the OB stage (E7.25), showing GFP-negative DE cells inserting between GFP-positive VE cells (used in Fig. 7c, d) (b) High magnification of E-CADHERIN channel, indicating two regions of data acquisition for a single egressing cell (red arrows inside pink squares). One measurement is taken on the cell membrane section facing the surface of the embryo, and the other is taken on the cell membrane section facing the embryo cavity. (c) Measurement of the cell membrane section facing the surface of the embryo of an egressing cell, used to generate the intensity graph (d) and Supplementary Table 1. (d) Graph of immuno-fluorescent signal intensity for region defined in (c). (e and f) Example of two measurements for a single inner cell; one measurement is taken on the cell membrane section facing the surface of the embryo, and the other is taken on the cell membrane section facing the embryo cavity. ACTIN signal facilitates visualization of the cell membrane. See also Supplementary Table 1.
Supplementary information
Supplementary Information
Supplementary Information (PDF 3080 kb)
Selective labelling of cells within the primitive streak of a mouse embryo.
360 degree rotational views of a longitudinal half of an Afp::GFP VE-reporter embryo. The embryo was electroporated with an RFP-expressing plasmid. Cells of the posterior epiblast adjacent to the primitive streak express RFP, and display a columnar morphology. The entire surface of the embryo expresses GFP, indicating that emVE dispersal has not yet begun. A, anterior; D, distal; P, posterior; Pr, proximal. (MOV 3365 kb)
Time-lapse depicting migration of mesoderm and DE cells.
Internal views of the embryo in Video S1, imaged over 8 hours. The RFP-positive columnar cells at the primitive streak change morphology becoming mesenchymal and progressively migrate anteriorly. A, anterior; D, distal; P, posterior; Pr, proximal. (MOV 2280 kb)
Time-lapse displaying egression of DE cells into the emVE.
Surface views of embryo in Video S2, initially depicting a uniform GFP-positive layer (the emVE). The emVE is progressively dispersed, and over time an increasing number of RFP-expressing cells appear on the surface of the embryo (white arrowheads). A, anterior; D, distal; L, left; P, posterior; Pr, proximal; R, right. (MOV 726 kb)
Time-lapse displaying migration and division of DE cells.
Surface view of Afp::GFP embryo electroporated with CAG::RFP. The second segment tracks a labeled cell migrating anteriorly, moving into a GFP-negative area of the VE, and dividing. (MOV 3227 kb)
Time-lapse displaying migration of cells in the mesodermal wings.
Afp::GFP embryo electroporated with CAG::RFP. The first segment shows green and red channels. Note that the GFP-positive VE layer remains uninterrupted for the duration of the time-lapse, suggesting that no egression has yet taken place. Second segment shows red channel and brightfield merge. (MOV 3671 kb)
The wild-type OB stage (E7.25) embryo has two BMs, while the Sox17 mutant only has one.
High magnification views of LAMA-1 localization in wild-type and Sox17 mutant embryos expressing the Afp::GFP VE-reporter. The wild-type displays two BMs, one at the epiblast-mesoderm interface and another at the mesoderm-endoderm interface. The Sox17 mutant exhibits a single BM, at the epiblast-mesoderm interface. end, endoderm; epi, epiblast; mes, mesoderm. (MOV 2795 kb)
Rights and permissions
About this article
Cite this article
Viotti, M., Nowotschin, S. & Hadjantonakis, AK. SOX17 links gut endoderm morphogenesis and germ layer segregation. Nat Cell Biol 16, 1146–1156 (2014). https://doi.org/10.1038/ncb3070
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb3070
This article is cited by
-
Coronary artery disease patient-derived iPSC-hepatocytes have distinct miRNA profile that may alter lipid metabolism
Scientific Reports (2023)
-
In vitro generation of transplantable insulin-producing cells from canine adipose-derived mesenchymal stem cells
Scientific Reports (2022)
-
Dominant role of DNA methylation over H3K9me3 for IAP silencing in endoderm
Nature Communications (2022)
-
In Parkinson's patient-derived dopamine neurons, the triplication of α-synuclein locus induces distinctive firing pattern by impeding D2 receptor autoinhibition
Acta Neuropathologica Communications (2021)
-
Transgenic fluorescent zebrafish lines that have revolutionized biomedical research
Laboratory Animal Research (2021)