Abstract
Chromosome breakage elicits transient silencing of ribosomal RNA synthesis, but the mechanisms involved remained elusive. Here we discover an in trans signalling mechanism that triggers pan-nuclear silencing of rRNA transcription in response to DNA damage. This is associated with transient recruitment of the Nijmegen breakage syndrome protein 1 (NBS1), a central regulator of DNA damage responses, into the nucleoli. We further identify TCOF1 (also known as Treacle), a nucleolar factor implicated in ribosome biogenesis and mutated in Treacher Collins syndrome, as an interaction partner of NBS1, and demonstrate that NBS1 translocation and accumulation in the nucleoli is Treacle dependent. Finally, we provide evidence that Treacle-mediated NBS1 recruitment into the nucleoli regulates rRNA silencing in trans in the presence of distant chromosome breaks.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Harper, J. W. & Elledge, S. J. The DNA damage response: ten years after. Mol. Cell 28, 739–745 (2007).
Shiloh, Y. & Ziv, Y. The ATM protein kinase: regulating the cellular response to genotoxic stress, and more. Nat. Rev. Mol. Cell Biol. 14, 197–210 (2013).
Difilippantonio, S. & Nussenzweig, A. The NBS1-ATM connection revisited. Cell Cycle 6, 2366–2370 (2007).
Chrzanowska, K. H., Gregorek, H., Dembowska-Bagińska, B., Kalina, M. A. & Digweed, M. Nijmegen breakage syndrome (NBS). Orphanet J. Rare Dis. 7, 13 (2012).
Stucki, M. & Jackson, S. P. γH2AX and MDC1: anchoring the DNA-damage-response machinery to broken chromosomes. DNA Repair 5, 534–543 (2006).
Spycher, C. et al. Constitutive phosphorylation of MDC1 physically links the MRE11-RAD50-NBS1 complex to damaged chromatin. J. Cell Biol. 181, 227–240 (2008).
Melander, F. et al. Phosphorylation of SDT repeats in the MDC1 N terminus triggers retention of NBS1 at the DNA damage-modified chromatin. J. Cell Biol. 181, 213–226 (2008).
Chapman, J. R. & Jackson, S. P. Phospho-dependent interactions between NBS1 and MDC1 mediate chromatin retention of the MRN complex at sites of DNA damage. EMBO Rep. 9, 795–801 (2008).
Wu, L., Luo, K., Lou, Z. & Chen, J. MDC1 regulates intra-S-phase checkpoint by targeting NBS1 to DNA double-strand breaks. Proc. Natl Acad. Sci. USA 105, 11200–11205 (2008).
Lloyd, J. et al. A supramodular FHA/BRCT-repeat architecture mediates Nbs1 adaptor function in response to DNA damage. Cell 139, 100–111 (2009).
Williams, R. S. et al. Nbs1 flexibly tethers Ctp1 and Mre11-Rad50 to coordinate DNA double-strand break processing and repair. Cell 139, 87–99 (2009).
Shanbhag, N. M., Rafalska-Metcalf, I. U., Balane-Bolivar, C., Janicki, S. M. & Greenberg, R. A. ATM-dependent chromatin changes silence transcription In cis to DNA double-strand breaks. Cell 141, 970–981 (2010).
Kruhlak, M. J. et al. The ATM repair pathway inhibits RNA polymerase I transcription in response to chromosome breaks. Nature 447, 730–734 (2007).
Lukas, C., Bartek, J. & Lukas, J. Imaging of protein movement induced by chromosomal breakage: tiny ‘local’ lesions pose great ‘global’ challenges. Chromosoma 114, 146–154 (2005).
Goldberg, M. et al. MDC1 is required for the intra-S-phase DNA damage checkpoint. Nature 421, 952–956 (2003).
Lukas, C. et al. Mdc1 couples DNA double-strand break recognition by Nbs1 with its H2AX-dependent chromatin retention. EMBO J. 23, 2674–2683 (2004).
Sakai, D. & Trainor, P. A. Treacher Collins syndrome: unmasking the role of Tcof1/treacle. Int. J. Biochem. Cell Biol. 41, 1229–1232 (2009).
Wise, C. A. et al. TCOF1 gene encodes a putative nucleolar phosphoprotein that exhibits mutations in Treacher Collins Syndrome throughout its coding region. Proc. Natl Acad. Sci. USA 94, 3110–3115 (1997).
Isaac, C. et al. Characterization of the nucleolar gene product, treacle, in Treacher Collins syndrome. Mol. Biol. Cell 11, 3061–3071 (2000).
Meier, U. T. & Blobel, G. Nopp140 shuttles on tracks between nucleolus and cytoplasm. Cell 70, 127–138 (1992).
Valdez, B. C., Henning, D., So, R. B., Dixon, J. & Dixon, M. J. The Treacher Collins syndrome (TCOF1) gene product is involved in ribosomal DNA gene transcription by interacting with upstream binding factor. Proc. Natl Acad. Sci. USA 101, 10709–10714 (2004).
Dixon, J. et al. Tcof1/Treacle is required for neural crest cell formation and proliferation deficiencies that cause craniofacial abnormalities. Proc. Natl Acad. Sci. USA 103, 13403–13408 (2006).
Pankotai, T., Bonhomme, C., Chen, D. & Soutoglou, E. DNAPKcs-dependent arrest of RNA polymerase II transcription in the presence of DNA breaks. Nat. Struct. Mol. Biol. 19, 276–282 (2012).
Matsuoka, S. et al. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science 316, 1160–1166 (2007).
Jones, N. C. et al. Prevention of the neurocristopathy Treacher Collins syndrome through inhibition of p53 function. Nat. Med. 14, 125–133 (2008).
Dauwerse, J. G. et al. Mutations in genes encoding subunits of RNA polymerases I and III cause Treacher Collins syndrome. Nat. Genet. 43, 20–22 (2010).
Ide, S., Miyazaki, T., Maki, H. & Kobayashi, T. Abundance of ribosomal RNA gene copies maintains genome integrity. Science 327, 693–696 (2010).
Lukas, C., Falck, J., Bartkova, J., Bartek, J. & Lukas, J. Distinct spatiotemporal dynamics of mammalian checkpoint regulators induced by DNA damage. Nat. Cell Biol. 5, 255–260 (2003).
Gudjonsson, T. et al. TRIP12 and UBR5 suppress spreading of chromatin ubiquitylation at damaged chromosomes. Cell 150, 697–709 (2012).
Nielsen, M. L. et al. Iodoacetamide-induced artifact mimics ubiquitination in mass spectrometry. Nat. Methods 5, 459–460 (2008).
Kelstrup, C. D., Young, C., Lavallee, R., Nielsen, M. L. & Olsen, J. V. Optimized fast and sensitive acquisition methods for shotgun proteomics on a quadrupole orbitrap mass spectrometer. J. Proteome Res. 11, 3487–3497 (2012).
Cox, J. & Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 26, 1367–1372 (2008).
Cox, J. et al. Andromeda: a peptide search engine integrated into the MaxQuant environment. J. Proteome Res. 10, 1794–1805 (2011).
Acknowledgements
We thank S. Jackson (The Gurdon Institute, Cambridge, UK) for reagents, R. Santoro for technical support in relation to rRNA transcription, C. Dinant for critical discussion of the FRAP data and members of the Department of Gynecology, the Novo Nordisk Foundation Center for Protein Research and the National Institute of Medical Research for helpful discussions. This work was supported by grants from the Swiss National Foundation (3100A0-111818 and 31003A-144284), Promedica Foundation, Lundbeckfonden (R93-A8863), Novo Nordisk Foundation and by the Kanton of Zürich. S.J.S. is supported by the Medical Research Council UK (U117584228).
Author information
Authors and Affiliations
Contributions
D.H.L. designed and performed most of the experiments, analysed data and contributed to writing the paper; F.H. designed the mass spectrometry screen that identified TCOF1 and performed some of the biochemical analysis; S.J. and M.L.N. designed and performed most of the mass spectrometry analysis, analysed data and contributed to writing the paper; J.A.C. and S.J.S. designed and performed the ITC experiments, analysed data and contributed to writing the paper; M.A. provided feedback in experimental design, performed some of the microscopic analysis and revised the written manuscript; L.I.T. provided feedback in experimental design and performed some of the microscopic analysis; K.G. performed some of the ChIP experiments; M. Gwerder purified proteins, generated reagents and performed in vitro phosphorylation assays and pulldown analysis; M-B.R. and M. Grøfte generated reagents; D.F. supervised the project; C.L. discovered NBS1 nucleolar localization and provided feedback in the design of the microscopic analysis and interpretation of the data; J.L. and M.S. coordinated and supervised the project, designed experiments, analysed data and wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 3 Additional data on rRNA silencing after DNA damage and NBS1 and MRE11 nucleolar localization.
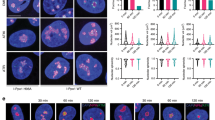
(a) Measurement of rRNA synthesis after laser-irradiation by 5-EU incorporation. A larger field of cells is shown here as compared to Fig. 1A (2 independent experiments; one representative image shown). (b) U2OS cells stably transfected with NBS1–GFP were presensitized with 10 μM BrdU for 24 h and exposed to laser micro-irradiation. To mark nucleoli, cells were immunostained with antibodies against Fibrillarin (2 independent experiments; 1 representative experiment shown). (c) Translocation of NBS1–GFP was followed by timelapse microscopy (5 independent experiments; 1 representative experiment shown). (d) U2OS cells stably transfected with GFP-MRE11 were presensitized with 10 μM BrdU for 24 h and exposed to laser micro-irradiation. Cells were immunostained with antibodies against γH2AX (2 independent experiments; 1 representative experiment shown). Scale bars, 10 μm.
Supplementary Figure 4 Measurement of rRNA synthesis after ionizing radiation by EU labelling.
(a) Dose titration of rRNA synthesis after ionizing radiation. Error bars represent s.e.m. (n = 3 independent experiments). (b) rRNA synthesis after ionizing radiation on depletion of MRE11 by two different siRNAs (measured by EU labelling 20 min after 5 Gy). The bars in the graph represent the average of 2 independent experiments.
Supplementary Figure 5 Measurement of rRNA synthesis after ionizing radiation and CPT treatment by qRT-PCR.
(a) rRNA synthesis after ionizing radiation and CPT treatment in the presence of ATM inhibitor (KU55933). Error bars represent s.e.m. (n = 4 independent experiments; samples were run in duplicates). (b) rRNA synthesis after ionizing radiation and CPT treatment on depletion of NBS1 by two different siRNAs. Error bars represent s.e.m. (n = 4 independent experiments for siNBS1-1; n = 3 independent experiments for siNBS1-2; all samples were run in duplicates). (c) rRNA synthesis after ionizing radiation and CPT treatment on depletion of MDC1. Error bars represent s.e.m. (n = 4 independent experiments; samples were run in duplicates).
Supplementary Figure 6 Treacle is heavily phosphorylated by CK2 in vitro.
Bacterially purified GST–Treacle fragments were incubated with recombinant CK2 and γ-[32P]-ATP, followed by SDS-PAGE and autoradiography. MDC1 fragments were used as positive and negative control, respectively 6. Note that fragment T-2 (aa 109-330) that contains the NBS1-interacting region is efficiently phosphorylated by CK2 in vitro (2 independent experiments; 1 representative experiment shown).
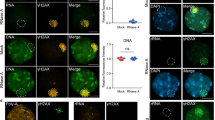
Supplementary Figure 7 Treacle localization and mobility in the nucleoli.
(a) Hela cells were immunostained with antibodies against Treacle and UBF (2 independent experiments; 1 representative experiment shown). Scale bar, 10 μm. (b) ChIP experiment with antibodies against mouse IgG or GFP in stably transfected U2OS GFP–Treacle cells. GAPDH was used as an unspecific target. Error bars represent s.e.m. (n = 4 independent experiments). (c) FRAP experiment in GFP–Treacle-expressing Hela cells. Bleach pulse was directed against one single nucleolus and fluorescence recovery was measured in the same nucleolus. T1/2 = 210 ± 40 s. The black line represents the median of 17 FRAP experiments included in the graph.
Supplementary Figure 8 Additional data about Treacle localization to sites of DNA damage.
(a) Schematic representation of Treacle-c. Note that the C-terminal region comprising the nucleolar localization signal is missing from this variant. (b) U2OS cells stably expressing GFP–Treacle-c were pre-sensitized with 10 μM BrdU for 24 h and exposed to laser micro-irradiation. Cells were treated with control siRNA and siRNA against NBS1 and immunostained with antibodies against γH2AX (2 independent experiments; 1 representative experiment shown). Scale bar, 10 μm (c) U2OS cells stably transfected with NBS1–GFP were pre-treated with PARP inhibitors and exposed to laser micro-irradiation. NBS1–GFP positive nucleoli were quantified. Error bars represent s.e.m. (n = 3 independent experiments; at least 45 cells with laser stripes probed for nucleolar NBS1 foci in each experiment, for statistical source data see Supplementary Table 2).
Supplementary Figure 9 Treacle phosphorylation in response to DNA damage.
293T cells were treated with the indicated siRNAs for 72 h and subsequently transiently transfected with HA-Treacle as indicated. 1 h before irradiation indicated samples were treated with 10 μM ATMi (KU55933). Cells were exposed to 10 Gy of ionizing radiation and immunoprecipitation was performed against HA (Treacle). Blots were probed with the indicated antibodies, including an antibody against pSQ (pS61 Bid; 2 independent experiments; 1 representative experiment shown).
Supplementary information
Supplementary Information
Supplementary Information (PDF 828 kb)
Rights and permissions
About this article
Cite this article
Larsen, D., Hari, F., Clapperton, J. et al. The NBS1–Treacle complex controls ribosomal RNA transcription in response to DNA damage. Nat Cell Biol 16, 792–803 (2014). https://doi.org/10.1038/ncb3007
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb3007
This article is cited by
-
SGF29 nuclear condensates reinforce cellular aging
Cell Discovery (2023)
-
Ribosome biosynthesis and Hedgehog activity are cooperative actionable signaling mechanisms in breast cancer following radiotherapy
npj Precision Oncology (2023)
-
rDNA Transcription in Developmental Diseases and Stem Cells
Stem Cell Reviews and Reports (2023)
-
TCOF1 upregulation in triple-negative breast cancer promotes stemness and tumour growth and correlates with poor prognosis
British Journal of Cancer (2022)
-
Mass spectrometry-based draft of the mouse proteome
Nature Methods (2022)