Abstract
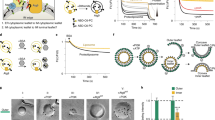
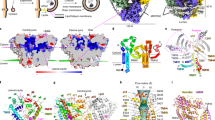
The components supporting autophagosome growth on the cup-like isolation membrane are likely to be different from those found on closed and maturing autophagosomes. The highly curved rim of the cup may serve as a functionally required surface for transiently associated components of the early acting autophagic machinery. Here we demonstrate that the E2-like enzyme, Atg3, facilitates LC3/GABARAP lipidation only on membranes exhibiting local lipid-packing defects. This activity requires an amino-terminal amphipathic helix similar to motifs found on proteins targeting highly curved intracellular membranes. By tuning the hydrophobicity of this motif, we can promote or inhibit lipidation in vitro and in rescue experiments in Atg3-knockout cells, implying a physiologic role for this stress detection. The need for extensive lipid-packing defects suggests that Atg3 is designed to work at highly curved membranes, perhaps including the limiting edge of the growing phagophore.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
06 June 2014
In the version of this Article originally published, the x axis labels 'WT' and 'K11L' on the graph in Fig. 5c were swapped. This error has now been corrected in all online versions of the Article.
04 July 2014
In all previously published versions of this Article, the graph in Fig. 5c was incorrect. This error has now been corrected in all online versions of the Article.
01 August 2014
Nat. Cell Biol. 16, 415–424 (2014); published online 20 April 2014; corrected after print 4 July 2014 In all previously published versions of this Article, the graph in Fig. 5c was incorrect; the correct graph is shown below. This error has now been corrected in all online versions of the Article.
References
Itakura, E. & Mizushima, N. Characterization of autophagosome formation site by a hierarchical analysis of mammalian Atg proteins. Autophagy 6, 764–776 (2010).
Ichimura, Y. et al. A ubiquitin-like system mediates protein lipidation. Nature 408, 488–492 (2000).
Kabeya, Y. et al. LC3, GABARAP and GATE16 localize to autophagosomal membrane depending on form-II formation. J. Cell Sci. 117, 2805–2812 (2004).
Nakatogawa, H., Ichimura, Y. & Ohsumi, Y. Atg8, a ubiquitin-like protein required for autophagosome formation, mediates membrane tethering and hemifusion. Cell 130, 165–178 (2007).
Weidberg, H. et al. LC3 and GATE-16/GABARAP subfamilies are both essential yet act differently in autophagosome biogenesis. EMBO J. 29, 1792–1802 (2010).
Shpilka, T., Weidberg, H., Pietrokovski, S. & Elazar, Z. Atg8: an autophagy-related ubiquitin-like protein family. Genome Biol. 12, 226 (2011).
Sou, Y. S. et al. The Atg8 conjugation system is indispensable for proper development of autophagic isolation membranes in mice. Mol. Biol. Cell 19, 4762–4775 (2008).
Komatsu, M. et al. Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J. Cell Biol. 169, 425–434 (2005).
Nishida, Y. et al. Discovery of Atg5/Atg7-independent alternative macroautophagy. Nature 461, 654–658 (2009).
Mizushima, N. et al. Dissection of autophagosome formation using Apg5-deficient mouse embryonic stem cells. J. Cell Biol. 152, 657–668 (2001).
Hayashi-Nishino, M. et al. A subdomain of the endoplasmic reticulum forms a cradle for autophagosome formation. Nat. Cell Biol. 11, 1433–1437 (2009).
Fan, W., Nassiri, A. & Zhong, Q. Autophagosome targeting and membrane curvature sensing by Barkor/Atg14(L). Proc. Natl Acad. Sci. USA 108, 7769–7774 (2011).
Drin, G. & Antonny, B. Amphipathic helices and membrane curvature. FEBS Letters 584, 1840–1847 (2010).
Hayashi-Nishino, M. et al. Electron tomography reveals the endoplasmic reticulum as a membrane source for autophagosome formation. Autophagy 6, 301–303 (2009).
Ichimura, Y. et al. In vivo and in vitro reconstitution of Atg8 conjugation essential for autophagy. J. Biol. Chem. 279, 40584–40592 (2004).
Sou, Y. S., Tanida, I., Komatsu, M., Ueno, T. & Kominami, E. Phosphatidylserine in addition to phosphatidylethanolamine is an in vitro target of the mammalian Atg8 modifiers, LC3, GABARAP, and GATE-16. J. Biol. Chem. 281, 3017–3024 (2006).
Tanida, I., Sou, Y. S., Minematsu-Ikeguchi, N., Ueno, T. & Kominami, E. Atg8L/Apg8L is the fourth mammalian modifier of mammalian Atg8 conjugation mediated by human Atg4B, Atg7 and Atg3. FEBS J. 273, 2553–2562 (2006).
Choy, A. et al. The Legionella effector RavZ inhibits host autophagy through irreversible Atg8 deconjugation. Science 338, 1072–1076 (2012).
Oh-oka, K., Nakatogawa, H. & Ohsumi, Y. Physiological pH and acidic phospholipids contribute to substrate specificity in lipidation of Atg8. J. Biol. Chem. 283, 21847–21852 (2008).
Brownell, J. E. et al. Substrate-assisted inhibition of ubiquitin-like protein-activating enzymes: the NEDD8 E1 inhibitor MLN4924 forms a NEDD8-AMP mimetic in situ. Mol. Cell 37, 102–111 (2010).
Nair, U. et al. SNARE proteins are required for macroautophagy. Cell 146, 290–302 (2011).
Zimmerberg, J. & Kozlov, M. M. How proteins produce cellular membrane curvature. Nat. Rev. Mol. Cell Biol. 7, 9–19 (2006).
Hatzakis, N. S. et al. How curved membranes recruit amphipathic helices and protein anchoring motifs. Nat. Chem. Biol. 5, 835–841 (2009).
Vamparys, L. et al. Conical lipids in flat bilayers induce packing defects similar to that induced by positive curvature. Biophys. J. 104, 585–593 (2013).
Vanni, S. et al. Amphipathic lipid packing sensor motifs: Probing bilayer defects with hydrophobic residues. Biophys. J. 104, 575–584 (2013).
Kamal, M. M., Mills, D., Grzybek, M. & Howard, J. Measurement of the membrane curvature preference of phospholipids reveals only weak coupling between lipid shape and leaflet curvature. Proc. Natl Acad. Sci. USA 106, 22245–22250 (2009).
Hope, M. J., Bally, M. B., Webb, G. & Cullis, P. R. Production of large unilamellar vesicles by a rapid extrusion procedure characterization of size distribution, trapped volume and ability to maintain a membrane potential. Biochim. Biophys. Acta. 812, 55–65 (1985).
Antonny, B. Mechanisms of membrane curvature sensing. Annu. Rev. Biochem. (2010).
Gautier, R., Douguet, D., Antonny, B. & Drin, G. HELIQUEST: a web server to screen sequences with specific alpha-helical properties. Bioinformatics 24, 2101–2102 (2008).
Radoshevich, L. et al. ATG12 conjugation to ATG3 regulates mitochondrial homeostasis and cell death. Cell 142, 590–600 (2010).
Hanada, T. et al. The Atg12-Atg5 conjugate has a novel E3-like activity for protein lipidation in autophagy. J. Biol. Chem. 282, 37298–37302 (2007).
Sakoh-Nakatogawa, M. et al. Atg12-Atg5 conjugate enhances E2 activity of Atg3 by rearranging its catalytic site. Nat. Struct. Mol. Biol. 20, 433–439 (2013).
Noda, N. N., Fujioka, Y., Hanada, T., Ohsumi, Y. & Inagaki, F. Structure of the Atg12-Atg5 conjugate reveals a platform for stimulating Atg8-PE conjugation. EMBO Rep. 14, 206–211 (2013).
Fujita, N. et al. The Atg16L complex specifies the site of LC3 lipidation for membrane biogenesis in autophagy. Mol. Biol. Cell 19, 2092–2100 (2008).
Kuma, A. et al. The role of autophagy during the early neonatal starvation period. Nature 432, 1032–1036 (2004).
Hosokawa, N., Hara, Y. & Mizushima, N. Generation of cell lines with tetracycline-regulated autophagy and a role for autophagy in controlling cell size. FEBS Lett. 580, 2623–2629 (2006).
Kaufmann, A., Beier, V., Franquelim, H. G. & Wollert, T. Molecular mechanism of autophagic membrane-scaffold assembly and disassembly. Cell 156, 469–481 (2014).
Fujioka, Y. et al. In vitro reconstitution of plant Atg8 and Atg12 conjugation systems essential for autophagy. J. Biol. Chem. 283, 1921–1928 (2008).
Hanada, T., Satomi, Y., Takao, T. & Ohsumi, Y. The amino-terminal region of Atg3 is essential for association with phosphatidylethanolamine in Atg8 lipidation. FEBS Lett. 583, 1078–1083 (2009).
Pranke, I. M. et al. alpha-Synuclein and ALPS motifs are membrane curvature sensors whose contrasting chemistry mediates selective vesicle binding. J. Cell Biol. 194, 89–103 (2011).
Bigay, J., Casella, J. F., Drin, G., Mesmin, B. & Antonny, B. ArfGAP1 responds to membrane curvature through the folding of a lipid packing sensor motif. EMBO J. 24, 2244–2253 (2005).
Sanjuan, M. A. et al. Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature 450, 1253–1257 (2007).
Yu, Z. Q. et al. Dual roles of Atg8-PE deconjugation by Atg4 in autophagy. Autophagy 8 (2012).
Nakatogawa, H., Ishii, J., Asai, E. & Ohsumi, Y. Atg4 recycles inappropriately lipidated Atg8 to promote autophagosome biogenesis. Autophagy 8, 177–186 (2012).
Proikas-Cezanne, T. & Robenek, H. Freeze-fracture replica immunolabelling reveals human WIPI-1 and WIPI-2 as membrane proteins of autophagosomes. J. Cell. Mol. Med. 15, 2007–2010 (2011).
Mizushima, N., Yoshimori, T. & Ohsumi, Y. The role of Atg proteins in autophagosome formation. Annu. Rev. Cell Dev. Biol. 27, 107–132 (2011).
Ragusa, M. J., Stanley, R. E. & Hurley, J. H. Architecture of the Atg17 complex as a scaffold for autophagosome biogenesis. Cell 151, 1501–1512 (2012).
Helfrich, W. Elastic properties of lipid bilayers: theory and possible experiments. Z. Naturforsch. C 28, 693–703 (1973).
Jotwani, A., Richerson, D. N., Motta, I., Julca-Zevallos, O. & Melia, T. J. Approaches to the study of Atg8-mediated membrane dynamics in vitro. Methods Cell Biol. 108, 93–116 (2012).
Choy, A. et al. The Legionella effector RavZ inhibits host autophagy through irreversible Atg8 deconjugation. Science 338, 1072–1076 (2012).
Nair, U. et al. SNARE proteins are required for macroautophagy. Cell 146, 290–302 (2011).
Jotwani, A., Richerson, D. N., Motta, I., Julca-Zevallos, O. & Melia, T. J. Approaches to the study of Atg8-mediated membrane dynamics in vitro. Methods Cell Biol. 108, 93–116 (2012).
Melia, T. J. et al. Regulation of membrane fusion by the membrane-proximal coil of the t-SNARE during zippering of SNAREpins. J. Cell Biol. 158, 929–940 (2002).
Burnette, W. N. ‘Western blotting’: Electrophoretic transfer of proteins from sodium dodecyl sulfate–polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal. Biochem. 112, 195–203 (1981).
Acknowledgements
Atg3-knockout MEFs were a gift from M. Komatsu, (Tokyo Metropolitan Organization for Medical Research, Japan) and Atg5 knockout MEFs from N. Mizushima (Tokyo University). This work was financially supported by grants from the NIH (GM100930—T.J.M.) and (NS063973—T.J.M. and A.Y.) and the ERC (advanced grant 268888—B.A.) and by Yale fellowships to G.P. (BioSTEP) and T.J.M. (Anderson). We also thank A. Jotwani and B. Mugo for help with electrophoresis experiments, E. Karatekin for help with dynamic light scattering and F. Pincet for helpful comments.
Author information
Authors and Affiliations
Contributions
S. Nath conceived, designed and carried out liposome experiments, analysed the data and assisted with a draft of the manuscript. J.D. conceived, designed and performed cell culture experiments and assisted with statistical analyses. V.S. conceived, designed and performed cell culture experiments and assisted with statistical analyses. G.P. made the initial discovery of curvature sensing in a liposomal system and carried out experiments with lipid composition tests. W.M.F. conceived, designed and carried out experiments testing activity in Atg5 knockout cell lines. S. Nag developed protein expression strategies and generated many of the tools supporting both the liposomal and cell culture studies. J.B. analysed imaging data. A.Y. conceived and designed experiments involved in Atg5 knockout rescues and assisted with the final manuscript. B.A. conceived and designed experiments on liposome curvature studies, assisted with data analysis and assisted with the manuscript. T.J.M. conceived the project, designed experiments, carried out liposome experiments and data analysis and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 In vitro observation of lipidation intermediate.
Lipidation of each LC3/GABARAP protein in vitro results in three distinct bands: the starting unlipidated protein, the faster migrating fully-lipidated protein, and a third band with a migration that depends upon the source protein (for LC3 and GRL2 it runs between the unlipidated and lipidated forms (Fig. 1), for GRL1 shown here, it runs slower than either form). To confirm that this additional product is a lipidation intermediate, a lipidation reaction was run as in Fig. 1 but without any final substrate lipid. By 28 minutes, the slowly migrating band can be observed. At this point, liposomes were added and the reaction was run for up to 100 additional minutes. By 120 min, essentially all of the slower migrating species has been consumed, suggesting that it is a reaction intermediate and not an off pathway end product.
Supplementary Figure 2 Mutations result in three classes of helical structures.
Amphipathic wheel representations of each of the mutants tested in Fig. 5 were generated with Heliquest29. Pictures demonstrate how the magnitude and direction of the hydrophobic moment (indicated with vectoral arrow) and the breadth of the hydrophobic surface (blue shading) is altered with each set of mutations.
Supplementary Figure 3 In rescued cells, LC3-II accumulation is increased upon higher Atg3 expression level (viral dose) and in the presence of Bafilomycin A1.
Cells as in Fig. 6a were infected with two different viral loads. The extent of rescue for Atg3 expression and LC3B lipidation is shown. The 5X condition is identical to the panel in Fig. 6a and represents the conditions used throughout the rest of the manuscript. Moreover, in cells rescued with wild-type Atg3 or K11L expression, LC3-II clears upon starvation. This clearance, blocked by Bafilomycin A1, shows that autophagic flux is restored in these cells.
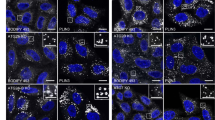
Supplementary Figure 4 Atg16 puncta intensities do not vary significantly with different Atg3 rescues.
a) representative images of Atg16L puncta in cells under full media, images as in Fig. 6b. b) – Average puncta intensity per puncta area is plotted. Intensities and areas were computationally identified with CellProfiler software as described in Methods. Error bars represent standard deviation. c) raw data (from Fig. 6d) of Atg16 puncta area/cell. For each Atg16 area per cell experiment, between seven and 14 images containing 5 to 20cellsper image were analyzed with the CellProfiler image analysis software to identify areas of intensity above a given threshold as described in Methods. N = 3 independent experiments for all samples except V15K for which n = 2. Precise cell numbers for each sample are given in Methods-statistics section.
Supplementary Figure 5 Atg16 and LC3 puncta numbers during starvation.
a) Representative images of starved cells. Both LC3 puncta and Atg16 puncta formation are shown. b) Atg16L puncta numbers do not significantly depend upon Atg3 expression or function during starvation. Cells as in Fig. 6d were starved before being subjected to fixation and immunolabeling with the anti-Atg16L antibody. Atg16L puncta area was obtained as described in Methods. Grey dashed line indicates the value for Atg3-/- cells rescued with wild-type Atg3. Atg3 variants that support lipidation of LC3 family proteins in vitro (Fig. 5) are indicated with black bars, while those that do not support lipidation are indicated with white bars. Error bars represent standard deviation. P-values are shown relative to rescue with WT (dashed line) *P < 0.05; **P < 0.1. c) Raw data (from Fig. 6c) of LC3 puncta in starvation – Bars are color-coded to indicate experiments conducted in parallel across samples. 22-98 cells were counted from at least two separate coverslips. Error bars represent standard deviation. Precise cell numbers for each sample are given in Methods-statistics section.
Supplementary Figure 6 Atg3 overexpression cannot rescue Atg5 deficiency.
A) Exogenous expression of K11V or K11W mutants of Atg3 cannot obviate requirement for Atg5 in LC3B lipidation. Atg3-/- or Atg5-/- MEFs were infected with lentiviruses encoding either the K11D, K11V or K11W Atg3 mutant. 48 hours post-infection, cells were treated with Bafilomycin A1 (Baf), then lysed and subjected to immunoblotting with the anti-LC3B and the anti-Atg3 antibodies. The asterisk indicates a non-specific band recognized by the anti-Atg3 antibody. B) Atg3 overexpression by transient transfection cannot rescue lipidation in Atg5 KO cells. Atg5 KO MEFs were transfected with mock, Atg3 WT or Atg3 K11W constructs, starved for 2 hours in 1X HBSS containing 10 mM HEPES and collected for Immunoblot analysis. Autophagosome maturation was impeded using the lysosome inhibitor chloroquine (10 M). C) Exogenously expressed Atg3 binds to endogenous Atg5 complexes. Overexpressed wild-type Atg3 with a COOH-terminal V5 tag can pull down endogenous Atg5 and Atg16. FT = flow through, T = total, IP = immunoprecipiate. D) V5-tagged Atg3 is functional. Atg3 used in IP (C), is able to rescue LC3-II formation in Atg3 KO cells, but only as long as the amphipathic helix is intact.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1645 kb)
Rights and permissions
About this article
Cite this article
Nath, S., Dancourt, J., Shteyn, V. et al. Lipidation of the LC3/GABARAP family of autophagy proteins relies on a membrane-curvature-sensing domain in Atg3. Nat Cell Biol 16, 415–424 (2014). https://doi.org/10.1038/ncb2940
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2940
This article is cited by
-
Control of NAD+ homeostasis by autophagic flux modulates mitochondrial and cardiac function
The EMBO Journal (2024)
-
Cyclic mechanical strain with high-tensile triggers autophagy in growth plate chondrocytes
Journal of Orthopaedic Surgery and Research (2022)
-
Macroautophagy in CNS health and disease
Nature Reviews Neuroscience (2022)
-
Eukaryotic initiation factor 5A2 mediates hypoxia-induced autophagy and cisplatin resistance
Cell Death & Disease (2022)
-
The caspase-6–p62 axis modulates p62 droplets based autophagy in a dominant-negative manner
Cell Death & Differentiation (2022)