Abstract
The Hippo–YAP pathway is an emerging signalling cascade involved in the regulation of stem cell activity and organ size. To identify components of this pathway, we performed an RNAi-based kinome screen in human cells. Our screen identified several kinases not previously associated with Hippo signalling that control multiple cellular processes. One of the hits, LKB1, is a common tumour suppressor whose mechanism of action is only partially understood. We demonstrate that LKB1 acts through its substrates of the microtubule affinity-regulating kinase family to regulate the localization of the polarity determinant Scribble and the activity of the core Hippo kinases. Our data also indicate that YAP is functionally important for the tumour suppressive effects of LKB1. Our results identify a signalling axis that links YAP activation with LKB1 mutations, and have implications for the treatment of LKB1-mutant human malignancies. In addition, our findings provide insight into upstream signals of the Hippo–YAP signalling cascade.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Change history
07 January 2014
In the version of this Article originally published, the name ‘Kwok-Kin Wong’ was spelled incorrectly in the author list. This has now been corrected in all online versions of the Article.
References
Takebe, N., Harris, P. J., Warren, R. Q. & Ivy, S. P. Targeting cancer stem cells by inhibiting Wnt, Notch, and Hedgehog pathways. Nat. Rev. Clin. Oncol. 8, 97–106 (2011).
Zhao, B., Lei, Q. Y. & Guan, K. L. The Hippo-YAP pathway: new connectionsbetween regulation of organ size and cancer. Curr. Opin. Cell Biol. 20, 638–646 (2008).
Ramos, A. & Camargo, F. D. The Hippo signaling pathway and stem cell biology. Trends Cell Biol. 22, 339–346 (2012).
Pan, D. The hippo signaling pathway in development and cancer. Dev. Cell 19, 491–505 (2010).
Ota, M. & Sasaki, H. Mammalian Tead proteins regulate cell proliferation and contact inhibition as transcriptional mediators of Hippo signaling. Development 135, 4059–4069 (2008).
Zhao, B. et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 22, 1962–1971 (2008).
Steinhardt, A. A. et al. Expression of Yes-associated protein in common solid tumors. Human Pathol. 39, 1582–1589 (2008).
Zhang, X. et al. The Hippo pathway transcriptional co-activator, YAP, is an ovarian cancer oncogene. Oncogene 30, 2810–2822 (2011).
Zhou, D. et al. Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the YAP oncogene. Cancer Cell 16, 425–438 (2009).
Bamford, S. et al. The COSMIC (Catalogue of Somatic Mutations in Cancer) database and website. Br. J. Cancer 19, 355–358 (2004).
Schlegelmilch, K. et al. YAP acts downstream of alpha-catenin to control epidermal proliferation. Cell 144, 782–795 (2011).
Zhang, N. et al. The Merlin/NF2 tumor suppressor functions through the YAP oncoprotein to regulate tissue homeostasis in mammals. Dev. Cell 19, 27–38 (2010).
Hamaratoglu, F. et al. The tumour-suppressor genes NF2/Merlin and Expanded act through Hippo signalling to regulate cell proliferation and apoptosis. Nat. Cell Biol. 8, 27–36 (2006).
Zhao, B. et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 21, 2747–2761 (2007).
Chen, F. JNK-induced apoptosis, compensatory growth, and cancer stem cells. Cancer Res. 72, 379–386 (2012).
Stark, M. S. et al. Frequent somatic mutations in MAP3K5 and MAP3K9 in metastatic melanoma identified by exome sequencing. Nat. Genet. 44, 165–169 (2012).
Schramek, D. et al. The stress kinase MKK7 couples oncogenic stress to p53 stability and tumor suppression. Nat. Genet. 43, 212–219 (2011).
Peifer, M. et al. Integrative genome analyses identify key somatic driver mutations of small-cell lung cancer. Nat. Genet. 44, 1104–1110 (2012).
Oricchio, E. et al. The Eph-receptor A7 is a soluble tumor suppressor for follicular lymphoma. Cell 147, 554–564 (2011).
Zaric, J. et al. Identification of MAGI1 as a tumor-suppressor protein induced by cyclooxygenase-2 inhibitors in colorectal cancer cells. Oncogene 31, 48–59 (2012).
Lee, D. W., Zhao, X., Yim, Y. I., Eisenberg, E. & Greene, L. E. Essential role of cyclin-G-associated kinase (Auxilin-2) in developing and mature mice. Mol. Biol. Cell 19, 2766–2776 (2008).
Doles, J. & Hemann, M. T. Nek4 status differentially alters sensitivity to distinct microtubule poisons. Cancer Res. 70, 1033–1041 (2010).
Boggiano, J. C., Vanderzalm, P. J. & Fehon, R. G. Tao-1 phosphorylates Hippo/MST kinases to regulate the Hippo-Salvador-Warts tumor suppressor pathway. Dev. Cell 21, 888–895 (2011).
Baas, A. F., Smit, L. & Clevers, H. LKB1 tumor suppressor protein: PARtaker in cell polarity. Trends Cell Biol. 14, 312–319 (2004).
Baas, A.F. et al. Complete polarization of single intestinal epithelial cells upon activation of LKB1 by STRAD. Cell 116, 457–466 (2004).
Nguyen, H. B., Babcock, J. T., Wells, C. D. & Quilliam, L. A. LKB1 tumor suppressor regulates AMP kinase/mTOR-independent cell growth and proliferation via the phosphorylation of YAP. Oncogene 32, 4100–4109 (2013).
Lizcano, J. M. et al. LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. EMBO J. 23, 833–843 (2004).
Hurov, J. & Piwnica-Worms, H. The Par-1/MARK family of protein kinases: from polarity to metabolism. Cell Cycle 6, 1966–1969 (2007).
Zhang, Y. et al. PAR-1 kinase phosphorylates Dlg and regulates itspostsynaptic targeting at the Drosophila neuromuscular junction. Neuron 53, 201–215 (2007).
Bilder, D., Li, M. & Perrimon, N. Cooperative regulation of cell polarity and growth by Drosophila tumor suppressors. Science 289, 113–116 (2000).
Yamanaka, T. & Ohno, S. Role of Lgl/Dlg/Scribble in the regulation of epithelial junction, polarity and growth. Front Biosci. 13, 6693–6707 (2008).
Grzeschik, N. A., Parsons, L. M., Allott, M. L., Harvey, K. F. & Richardson, H. E. Lgl, aPKC, and Crumbs regulate the Salvador/Warts/Hippo pathway through two distinct mechanisms. Curr. Biol. 20, 573–581 (2010).
Parsons, L. M., Grzeschik, N. A., Allott, M. L. & Richardson, H. E. Lgl/aPKC and Crb regulate the Salvador/Warts/Hippo pathway. Fly (Austin) 4, 288–293 (2010).
Cordenonsi, M. et al. The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell 147, 759–772 (2011).
Sanchez-Cespedes, M. A role for LKB1 gene in human cancer beyond the Peutz-Jeghers syndrome. Oncogene 26, 7825–7832 (2007).
Ji, H. et al. LKB1 modulates lung cancer differentiation and metastasis. Nature 448, 807–810 (2007).
Katajisto, P. et al. The LKB1 tumor suppressor kinase in human disease. Biochim. Biophys. Acta 1775, 63–75 (2007).
Zhou, W., Marcus, A. I. & Vertino, P. Dysregulation of mTOR activity through LKB1 inactivation. Chin. J. Cancer 32, 427–433 (2013).
Benton, R. & St Johnston, D. Drosophila PAR-1 and 14-3-3 inhibit Bazooka/PAR-3 to establish complementary cortical domains in polarized cells. Cell 115, 691–704 (2003).
Varelas, X. et al. The Crumbs complex couples cell density sensing to Hippo-dependent control of the TGF-beta-SMAD pathway. Dev. Cell 19, 831–844 (2010).
Sansores-Garcia, L. et al. Modulating F-actin organization induces organ growth by affecting the Hippo pathway. EMBO J. 30, 2325–2335 (2011).
Osmani, N., Vitale, N., Borg, J. P. & Etienne-Manneville, S. Scrib controls Cdc42 localization and activity to promote cell polarization during astrocyte migration. Curr. Biol. 16, 2395–2405 (2006).
Xu, X., Omelchenko, T. & Hall, A. LKB1 tumor suppressor protein regulates actin filament assembly through Rho and its exchange factor Dbl independently of kinase activity. BMC Cell Biol. 11, 77 (2010).
Morton, JP et al. LKB1 haploinsufficiency cooperates with Kras to promote pancreatic cancer through suppression of p21-dependent growth arrest. Gastroenterology 139, 586–597 (2010).
Stec, D. E., Davisson, R. L., Ha skell, R. E., Davidson, B. L. & Sigmund, CD. Efficient liver-specific deletion of a floxed human angiotensinogen transgene by adenoviral delivery of CRE-Recombinase in vivo. J. Biol. Chem. 274, 21285–21290 (1999).
Acknowledgements
We are grateful for stimulating and insightful discussions by members of the Camargo laboratory. Special thanks to R. Bronson of the Harvard Rodent Facility and R. Mathieu of the Stem Cell Program FACS facility. We are grateful to N. Bardeesy for Lkb1 conditional mice. This study was supported by awards from Stand Up to Cancer-AACR initiative (F.D.C.), NIH grant R01 CA131426 (F.D.C.) and DOD 81XWH-10-1-0724. M.M. is a DOD-CDMRP Postdoctoral Fellow. F.D.C. is a Pew Scholar in the Biomedical Sciences.
Author information
Authors and Affiliations
Contributions
M.M. and F.D.C. designed the study and wrote the paper. M.M., J.S., A.L. and S.C. performed the experimental work. J.G., V.L.F., C.W. and M.F. provided human clinical tissue. O.S., K-K.W. and C.K. provided mouse tumour samples. C.K. and K-K.W. analysed the data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
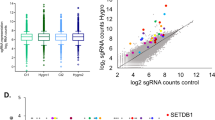
Supplementary Figure 1 Identification of kinases that can modulate the Hippo Signaling Pathway in vitro.
(a) Immunofluorescence for YAP localization in confluent HaCaT cells following siRNA transfection of selected kinase hits. Scale bars, 200 μm. Experiment was repeated 3 times independently (b) Western Blot for YAP S127 phosphorylation, and total YAP in 293T cells four days following siRNA transfection of selected kinase hits. Representative blot is shown. This experiment was repeated 3 independent times (c) Mitogen activated kinase pathway siRNA mini-screen using the STBS-luciferase reporter in 293T cells compared to scrambled control (CTR). Data shown is from technical triplicates. The experiment was repeated in three independent experiments. 5 bD) Selective JNK small molecule screen using the STBS-luciferase reporter in 293T cells. Schematic demonstrates where the inhibitors function. Fold changes calculated are compared to untreated controls.
Supplementary Figure 2 LKB1 acts upstream of the Hippo Signaling Pathway in vitro and in vivo.
(a) Knockdown of LKB1 using multiple different siRNA oligos increases STBS-luciferase reporter activity in 293T cells. n = 5 biological replicates ± SD. (b) Knockdown of LKB1 reproducibly increases STBS-luciferase reporter activity across various cell lines. n = 3 biological replicates. Error bars represent mean ± SD. (c) Knockdown of LKB1 reproducibly decreases YAP S127 phosphorylation across various cell lines by western blot analysis. (d) Knockdown of LKB1 in DLD1 cells promotes YAP nuclear localization at confluent cell densities. Experiment has been performed independently three times. Scale bar, 200 μm (e) qPCR validation of LKB1 and YAP siRNA knockdown in 293T cells. n = 3 biological replicates, ± SD. (f) LKB1 activation in W4 cells repressed TEAD-reporter activation. n = 3 replicates per biological triplicate (g) Decreased activity of MST1/2 in LKB1 deficient livers. Ratio of cleaved MST1 versus full length MST is shown for an average from n = 4 mice. (h) Quantification of YAP localization in W4 cells following MST1/2 or LATS1/2 knockdown. Data are derived from three independent experiments where at least 300 cells where scored. N = 3, error bars represent mean ± SD. (i) Validation of MST1/2 and LATS2 downstream of LKB1 using two additional sets of siRNAS. Representative figure from three independent experiments is shown. Scale bars, 20 μm (j, k) Immunoprecipitation of overexpressed LKB1, LATS1, and MST1 in 293T cells. Representative blot is shown. Experiment has been performed three times independently. Error bars represent mean ± SD from triplicate samples. **P<0.01, two-tailed t-test.
Supplementary Figure 3 Yap1 activity and localization is dependent on the LKB1 substrate, MARKs.
(a) qPCR validation of siRNA knockdown of MARK1 and MARK4 and expression of YAP-target genes, CTGF and CYR61. n = 3 biological replicates (b) STBS reporter activity following knockdown of MARK4 and YAP in 293T cells. n = 3 biological replicates (c) Western blot showing expression level of Mob1/LATS1/LATS2 in MARK4 knockdown cells. Representative blot is shown from three independent experiments (d) Activation of LKB1 in W4 cells promotes activation of MARKs as measured by Thr215 phosphorylation (MARK1) in the kinase activation loop. Representative blot is shown from three independent experiments (e) Immunofluorescence for YAP localization (green) and cell polarization (red) in doxycycline-untreated W4 cells following knockdown of MARK4. N = 3 biological replicates. Each experiment was performed with technical triplicates. Scale bar, 20 μm. (f) YAP localization in W4 cells following LKB1 activation and knockdown of MARK4 using 2 independent siRNA’s. Three independent experiments were performed. Scale bars, 20 μm. (g) Biochemical analysis of phospho-YAP localization by subcellular fractionation of W4 cells treated with doxycycline and MARK knockdown. Representative blot from three independent experiments is shown. Error bars represent mean ± SD.
Supplementary Figure 4 Scribble expression and correct localization is required for Yap1 activity.
(a) Immunofluorescence for Scribble (SCRIB) localization (green) in DLD1 cells following LKB1 and MARK siRNA knockdown. Experiment was repeated three times. Scale bar, 200 μm. (b-c) Immunoblot and qPCR for SCRIB expression following LKB1 and MARK1 knockdown in MCF7 cells. n = 3 biological triplicates (d) siRNA knockdown of SCRIB in 293T cells induces TEAD-reporter activity. n = 3 independent experiments. (e) qPCR validation of SCRIB knockdown using 3 independent siRNA’s. n = 3 independent experiments. (f) Immunofluorescence for YAP localization (green) and cell polarization (red) in doxycycline-untreated W4 cells following knockdown of SCRIB. Biological replicates were performed three times. Scale bar, 20 μm. (g) Quantification of YAP localization in W4 cells following scribble knockdown. Data are derived from three independent experiments where at least 300 cells where scored. (h) Immunofluorescence for YAP localization following activation of LKB1 and knockdown of scribble using 2 independent siRNA’s. Scale bar 20 μm. (i) Immunoprecipitation of overexpressed MARK4 with SCRIB, LKB1, MST1, and LATS1. Experiment was repeated three times. (j) Immunoprecipitated SCRIB in MARK1-knockdown cells show decreased interaction with MST and LATS. Experiment was repeated three times. Error bars represent mean ± SD.
Supplementary Figure 5 Generation of a Hippo Gene Expression Signature and localization of Yap in human LKB1-mutant tissues.
(a) Microarray analysis on three different cancer cell lines reveal a core set of genes whose expression changes following siRNA knockdown of NF2 + LATS1/2, and for which this response is dependent on YAP/TAZ. Differentially expressed genes were defined as those with at least 2 fold change in NF2/LATS2 double knockdown cells and a p value smaller than 0.05. (b) YAP immunohistochemistry on human SMAD4 juvenille polyposis (JP) intestinal polyp compared to a human LKB1 mutant Peutz-Jeghers (PJ) intestinal polyp. Scale bar, 500 μm.
Supplementary Figure 6 Yap1 acts downstream of LKB1 and can overcome LKB1 tumor suppressive function.
(a) Quantification of triplicate samples of number and size of W4, W4TetOYAP, +/- doxycycline colonies in soft agar assays. n = 3 biological triplicates (b) Tumor weights of W4, W4TetOYAP, +/- doxycycline after seven weeks. Representative images of nude mice carrying xenografts with W4, W4TetOYAP tumors. N = 7 mice per each of the four genotypes. (c) qPCR validation of shRNA knockdown of LATS2 and activation of YAP-target genes in W4 cells. N = 3 biological replicates. (d) qPCR of Scribble following Scribble siRNA knockdown in the presence of LKB1 activation. n = 3 biological replicates. Error bars represent mean ± SD.
Supplementary Figure 7 Loss of Yap1 in LKB1 mutant xenograft show tumor growth inhibition.
(a) Quantification of triplicate samples of number and size of W4, W4TetOYAP, +/- doxycycline colonies. n = 3 biological replicates (b-c) Average tumor size and number per mouse following intravenous injection of 1×106 A549iYAPshRNA cells (-/+ doxycycline) for 6 weeks. n = 3 mice per group (d-f) Two cell lines that are low in YAP target gene expression were infected with shYAP1 hairpins and assessed for proliferation using MTS assays (e) and soft agar colony formation assay (f) n = 3 biological replicates. Scale bar, 500 μm. (g) Expression levels of LKB1 and YAP following Ad-cre administration in Lkb1-floxed and Lkb1/YAP floxed livers. n = 4 mice per genotype. Error bars represent mean ± SD.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1080 kb)
Supplementary Table 1
Supplementary Information (XLSX 43 kb)
Supplementary Table 2
Supplementary Information (XLSX 36 kb)
Rights and permissions
About this article
Cite this article
Mohseni, M., Sun, J., Lau, A. et al. A genetic screen identifies an LKB1–MARK signalling axis controlling the Hippo–YAP pathway. Nat Cell Biol 16, 108–117 (2014). https://doi.org/10.1038/ncb2884
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2884
This article is cited by
-
STK11 loss leads to YAP1-mediated transcriptional activation in human KRAS-driven lung adenocarcinoma cell lines
Cancer Gene Therapy (2024)
-
Clinicopathological and prognostic significance of LKB1 expression in gastric cancer: a systematic review and meta-analysis
Scientific Reports (2023)
-
Non-hippo kinases: indispensable roles in YAP/TAZ signaling and implications in cancer therapy
Molecular Biology Reports (2023)
-
Activated TAZ induces liver cancer in collaboration with EGFR/HER2 signaling pathways
BMC Cancer (2022)
-
MARK2 regulates chemotherapeutic responses through class IIa HDAC-YAP axis in pancreatic cancer
Oncogene (2022)