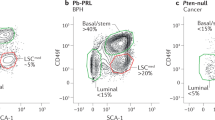
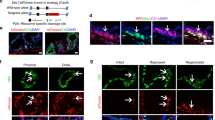
Abstract
The prostate is a glandular epithelium composed of basal, luminal and neuroendocrine cells that originate from the urogenital sinus during embryonic development. After birth, the prostate keeps developing until the end of puberty. Here, we used inducible genetic lineage tracing experiments in mice to investigate the cellular hierarchy that governs prostate postnatal development. We found that prostate postnatal development is mediated by basal multipotent stem cells that differentiate into basal, luminal and neuroendocrine cells, as well as by unipotent basal and luminal progenitors. Clonal analysis of basal cells revealed the existence of bipotent and unipotent basal progenitors as well as basal cells already committed to the luminal lineage with intermediate cells co-expressing basal and luminal markers associated with this commitment step. The existence of multipotent basal progenitors during prostate postnatal development contrasts with the distinct pools of unipotent basal and luminal stem cells that mediate adult prostate regeneration. Our results uncover the cellular hierarchy acting during prostate development and will be instrumental in defining the cellular origin and the mechanisms underlying prostate cancer initiation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Timms, B. G. Prostate development: a historical perspective. Differentiation 76, 565–577 (2008).
Cunha, G. R. et al. The endocrinology and developmental biology of the prostate. Endocr. Rev. 8, 338–362 (1987).
Shen, M. M. & Abate-Shen, C. Molecular genetics of prostate cancer: new prospects for old challenges. Genes Dev. 24, 1967–2000 (2010).
Kasper, S. Exploring the origins of the normal prostate and prostate cancer stem cell. Stem Cell Rev. 4, 193–201 (2008).
Signoretti, S. et al. p63 is a prostate basal cell marker and is required for prostate development. Am. J. Pathol. 157, 1769–1775 (2000).
Lilja, H. & Abrahamsson, P. A. Three predominant proteins secreted by the human prostate gland. Prostate 12, 29–38 (1988).
Peehl, D. M., Sellers, R. G. & McNeal, J. E. Keratin 19 in the adult human prostate: tissue and cell culture studies. Cell Tissue Res. 285, 171–176 (1996).
Xue, Y., Smedts, F., Debruyne, F. M., de la Rosette, J. J. & Schalken, J. A. Identification of intermediate cell types by keratin expression in the developing human prostate. Prostate 34, 292–301 (1998).
Wang, Y., Hayward, S., Cao, M., Thayer, K. & Cunha, G. Cell differentiation lineage in the prostate. Differentiation 68, 270–279 (2001).
Hudson, D. L. et al. Epithelial cell differentiation pathways in the human prostate: identification of intermediate phenotypes by keratin expression. J. Histochem. Cytochem. 49, 271–278 (2001).
Taylor, R. A., Toivanen, R. & Risbridger, G. P. Stem cells in prostate cancer: treating the root of the problem. Endocr. Relat. Cancer 17, R273–R285 (2010).
Marker, P. C., Donjacour, A. A., Dahiya, R. & Cunha, G. R. Hormonal, cellular, and molecular control of prostatic development. Dev. Biol. 253, 165–174 (2003).
Staack, A., Donjacour, A. A., Brody, J., Cunha, G. R. & Carroll, P. Mouse urogenital development: a practical approach. Differentiation 71, 402–413 (2003).
Blanpain, C., Horsley, V. & Fuchs, E. Epithelial stem cells: turning over new leaves. Cell 128, 445–458 (2007).
Cunha, G. R. Mesenchymal-epithelial interactions: past, present, and future. Differentiation 76, 578–586 (2008).
Cunha, G. R., Chung, L. W., Shannon, J. M., Taguchi, O. & Fujii, H. Hormone-induced morphogenesis and growth: role of mesenchymal-epithelial interactions. Recent Prog. Horm Res. 39, 559–598 (1983).
Wu, C. T. et al. Increased prostate cell proliferation and loss of cell differentiation in mice lacking prostate epithelial androgen receptor. Proc. Natl Acad. Sci. USA 104, 12679–12684 (2007).
Sugimura, Y., Cunha, G. R. & Donjacour, A. A. Morphogenesis of ductal networks in the mouse prostate. Biol. Reprod. 34, 961–971 (1986).
Yang, A. et al. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature 398, 714–718 (1999).
Mills, A. A. et al. p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature 398, 708–713 (1999).
Kurita, T., Medina, R. T., Mills, A. A. & Cunha, G. R. Role of p63 and basal cells in the prostate. Development 131, 4955–4964 (2004).
Signoretti, S. et al. p63 regulates commitment to the prostate cell lineage. Proc. Natl Acad. Sci. USA 102, 11355–11360 (2005).
English, H. F., Santen, R. J. & Isaacs, J. T. Response of glandular versus basal rat ventral prostatic epithelial cells to androgen withdrawal and replacement. Prostate 11, 229–242 (1987).
Evans, G. S. & Chandler, J. A. Cell proliferation studies in the rat prostate: II. The effects of castration and androgen-induced regeneration upon basal and secretory cell proliferation. Prostate 11, 339–351 (1987).
Xin, L., Lukacs, R. U., Lawson, D. A., Cheng, D. & Witte, O. N. Self-renewal and multilineage differentiation in vitro from murine prostate stem cells. Stem Cells 25, 2760–2769 (2007).
Goldstein, A. S. et al. Trop2 identifies a subpopulation of murine and human prostate basal cells with stem cell characteristics. Proc. Natl Acad. Sci. USA 105, 20882–20887 (2008).
Leong, K. G., Wang, B. E., Johnson, L. & Gao, W.Q. Generation of a prostate from a single adult stem cell. Nature 456, 804–808 (2008).
Lawson, D. A., Xin, L., Lukacs, R. U., Cheng, D. & Witte, O. N. Isolation and functional characterization of murine prostate stem cells. Proc. Natl Acad. Sci. USA 104, 181–186 (2007).
Wang, X. et al. A luminal epithelial stem cell that is a cell of origin for prostate cancer. Nature 461, 495–500 (2009).
Liu, J. et al. Regenerated luminal epithelial cells are derived from preexisting luminal epithelial cells in adult mouse prostate. Mol. Endocrinol. 25, 1849–1857 (2011).
Choi, N., Zhang, B., Zhang, L., Ittmann, M. & Xin, L. Adult murine prostate basal and luminal cells are self-sustained lineages that can both serve as targets for prostate cancer initiation. Cancer Cell 21, 253–265 (2012).
Van Keymeulen, A. et al. Distinct stem cells contribute to mammary gland development and maintenance. Nature 479, 189–193 (2011).
Blackwood, J. K. et al. In situ lineage tracking of human prostatic epithelial stem cell fate reveals a common clonal origin for basal and luminal cells. J. Pathol. 225, 181–188 (2011).
Gaisa, N. T. et al. Clonal architecture of human prostatic epithelium in benign and malignant conditions. J. Pathol. 225, 172–180 (2011).
Goldstein, A. S., Huang, J., Guo, C., Garraway, I. P. & Witte, O. N. Identification of a cell of origin for human prostate cancer. Science 329, 568–571 (2010).
Trotman, L. C. et al. Pten dose dictates cancer progression in the prostate. PLoS Biol. 1, E59 (2003).
Wang, S. et al. Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell 4, 209–221 (2003).
Ratnacaram, C. K. et al. Temporally controlled ablation of PTEN in adult mouse prostate epithelium generates a model of invasive prostatic adenocarcinoma. Proc. Natl Acad. Sci. USA 105, 2521–2526 (2008).
Srinivas, S. et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 1, 4–11 (2001).
Nguyen, H., Rendl, M. & Fuchs, E. Tcf3 governs stem cell features and represses cell fate determination in skin. Cell 127, 171–183 (2006).
Perl, A. K., Wert, S. E., Nagy, A., Lobe, C. G. & Whitsett, J. A. Early restriction of peripheral and proximal cell lineages during formation of the lung. Proc. Natl Acad. Sci. USA 99, 10482–10487 (2002).
Van Keymeulen, A. et al. Epidermal progenitors give rise to Merkel cells during embryonic development and adult homeostasis. J. Cell Biol. 187, 91–100 (2009).
Acknowledgements
We thank our colleagues who provided us with reagents, which are cited in the text. We thank our colleagues from the Blanpain laboratory and C. Govaerts for their comments on the manuscript and J-M. Vanderwinden and F. Bollet-Quivogne for their help with confocal imaging. C.B. and A.V.K. are chercheur qualifié, M.O. is a collaborateur scientifique of the FRS/FNRS. C.B. is an investigator of WELBIO. This work was supported by the FNRS, TELEVIE, the program d’excellence CIBLES of the Wallonia Region, a research grant from the Fondation Contre le Cancer, the ULB fondation, the fond Gaston Ithier, a starting grant of the European Research Council (ERC) and the EMBO Young Investigator Program.
Author information
Authors and Affiliations
Contributions
M.O., A.V.K. and C.B. designed the experiments and performed data analysis. M.O. and A.V.K. performed most of the experiments. B.D.S. performed mathematical modelling and data analysis. Y.A. generated K8CREER transgenic mice. G.B. and N.S. provided technical support. M.O. and A.V.K. prepared the figures. C.B. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 721 kb)
Supplementary Note
Supplementary Information (PDF 158 kb)
Supplementary Table 1
Supplementary Information (XLSX 10 kb)
Supplementary Table 2
Supplementary Information (XLSX 13 kb)
Supplementary Table 3
Supplementary Information (XLSX 11 kb)
Supplementary Table 4
Supplementary Information (XLSX 9 kb)
Supplementary Table 5
Supplementary Information (XLSX 10 kb)
Supplementary Table 6
Supplementary Information (XLSX 10 kb)
Supplementary Table 7
Supplementary Information (XLSX 10 kb)
Supplementary Table 8
Supplementary Information (XLSX 10 kb)
Supplementary Table 9
Supplementary Information (XLSX 10 kb)
Supplementary Table 10
Supplementary Information (XLSX 12 kb)
Supplementary Table 11
Supplementary Information (XLSX 9 kb)
Rights and permissions
About this article
Cite this article
Ousset, M., Van Keymeulen, A., Bouvencourt, G. et al. Multipotent and unipotent progenitors contribute to prostate postnatal development. Nat Cell Biol 14, 1131–1138 (2012). https://doi.org/10.1038/ncb2600
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2600
This article is cited by
-
YAP is required for prostate development, regeneration, and prostate stem cell function
Cell Death Discovery (2023)
-
The testosterone paradox of advanced prostate cancer: mechanistic insights and clinical implications
Nature Reviews Urology (2023)
-
Prostate zones and cancer: lost in transition?
Nature Reviews Urology (2022)
-
Prostate luminal progenitor cells: from mouse to human, from health to disease
Nature Reviews Urology (2022)
-
Stromal androgen signaling acts as tumor niches to drive prostatic basal epithelial progenitor-initiated oncogenesis
Nature Communications (2022)