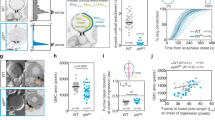
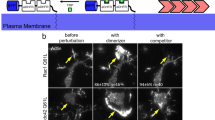
Abstract
The biological impact of Rho depends critically on the precise subcellular localization of its active, GTP-loaded form. This can potentially be determined by the balance between molecules that promote nucleotide exchange or GTP hydrolysis. However, how these activities may be coordinated is poorly understood. We now report a molecular pathway that achieves exactly this coordination at the epithelial zonula adherens. We identify an extramitotic activity of the centralspindlin complex, better understood as a cytokinetic regulator, which localizes to the interphase zonula adherens by interacting with the cadherin-associated protein, α-catenin. Centralspindlin recruits the RhoGEF, ECT2, to activate Rho and support junctional integrity through myosin IIA. Centralspindlin also inhibits the junctional localization of p190 B RhoGAP, which can inactivate Rho. Thus, a conserved molecular ensemble that governs Rho activation during cytokinesis is used in interphase cells to control the Rho GTPase cycle at the zonula adherens.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Jaffe, A. B. & Hall, A. Rho GTPases: Biochemistry and biology. Annu. Rev. Cell. Dev. Biol. 21, 247–269 (2005).
Bos, J. L., Rehmann, H. & Wittinghofer, A. GEFs and GAPs: critical elements in the control of small G proteins. Cell 129, 865–877 (2007).
Miller, A. L. & Bement, W. M. Regulation of cytokinesis by Rho GTPase flux. Nat. Cell Biol. 11, 71–77 (2009).
Pertz, O., Hodgson, L., Klemke, R. L. & Hahn, K. M. Spatiotemporal dynamics of RhoA activity in migrating cells. Nature 440, 1069–1072 (2006).
Yonemura, S., Hirao-Minakuchi, K. & Nishimura, Y. Rho localization in cells and tissues. Exp. Cell Res. 295, 300–314 (2004).
Yoshizaki, H. et al. Activity of Rho-family GTPases during cell division as visualized with FRET-based probes. J. Cell Biol. 162, 223–232 (2003).
Yamada, S. & Nelson, W. J. Localized zones of Rho and Rac activities drive initiation and expansion of epithelial cell–cell adhesion. J. Cell Biol. 178 (2007).
Bement, W. M., Miller, A. L. & von Dassow, G. Rho GTPase activity zones and transient contractile arrays. Bioessays 28, 983–993 (2006).
Yuce, O., Piekny, A. & Glotzer, M. An ECT2-central spindlin complex regulates the localization and function of RhoA. J. Cell Biol. 170, 571–582 (2005).
Magie, C. R., Pinto-Santini, D. & Parkhurst, S. M. Rho1 interacts with p120ctn and α-catenin, and regulates cadherin-based adherens junction components in Drosophila. Development 129, 3771–3782 (2002).
Takaishi, K., Sasaki, T., Kotani, H., Nishioka, H. & Takai, Y. Regulation of cell–cell adhesion by Rac and Rho small G proteins in MDCK cells. J. Cell Biol. 139, 1047–1059 (1997).
Braga, V. M. M., Machesky, L. M., Hall, A. & Hotchin, N. A. The small GPTases rho and rac are required for the formation of cadherin-dependent cell–cell contacts. J. Cell Biol. 137, 1421–1431 (1997).
Smutny, M. et al. Myosin II isoforms identify distinct functional modules that support integrity of the epithelial zonula adherens. Nat. Cell Biol. 12, 696–702 (2010).
Carramusa, L., Ballestrem, C., Zilberman, Y. & Bershadsky, A. D. Mammalian diaphanous-related formin Dia1 controls the organization of E-cadherin-mediated cell–cell junctions. J. Cell Sci. 120, 3870–3882 (2007).
Meng, W., Mushika, Y., Ichii, T. & Takeichi, M. Anchorage of microtubule minus ends to adherens junctions regulates epithelial cell–cell contacts. Cell 135, 948–959 (2008).
Kametani, Y. & Takeichi, M. Basal-to-apical cadherin flow at cell junctions. Nat. Cell Biol. 9, 92–98 (2007).
Wolfe, B. A. & Glotzer, M. Single cells (put a ring on it). Genes Dev. 23, 896–901 (2009).
Stehbens, S. J. et al. Dynamic microtubules regulate the local concentration of E-cadherin at cell–cell contacts. J. Cell Sci. 119, 1801–1811 (2006).
Jordan, M. A. & Wilson, L. Use of drugs to study role of microtubule assembly dynamics in living cells. Methods Enzymol. 298, 252–276 (1998).
Perez, F., Diamantopoulos, G. S., Stalder, R. & Kreis, T. E. CLIP-170 highlights growing microtubule ends in vivo. Cell 96, 517–527 (1999).
Shewan, A. M. et al. Myosin 2 is a key rho kinase target necessary for the local concentration of E-Cadherin at cell–cell contacts. Mol. Biol. Cell 16, 4531–4532 (2005).
Komarova, Y. et al. Mammalian end binding proteins control persistent microtubule growth. J. Cell Biol. 184, 691–706 (2009).
Komarova, Y. et al. EB1 and EB3 control CLIP dissociation from the ends of growing microtubules. Mol. Biol. Cell 16, 5334–5345 (2005).
Tatsumoto, T., Xie, X., Blumenthal, R., Okamoto, I. & Miki, T. Human ECT2 is an exchange factor for Rho GTPases, phosphorylated in G2/M phases, and involved in cytokinesis. J. Cell Biol. 147, 921–928 (1999).
Somers, W. G. & Saint, R. A RhoGEF and Rho family GTPase-activating protein complex link the contractile ring to cortical microtubules at the onset of cytokinesis. Dev. Cell 4, 29–39 (2003).
Liu, X. F., Ishida, H., Raziuddin, R. & Miki, T. Nucleotide exchange factor ECT2 interacts with the polarity protein complex Par6/Par3/protein kinase Czeta (PKCzeta) and regulates PKCzeta activity. Mol. Cell. Biol. 24, 6665–6675 (2004).
Sawyer, J. M. et al. Apical constriction: a cell shape change that can drive morphogenesis. Dev. Biol. 341, 5–19 (2010).
Miyake, Y. et al. Actomyosin tension is required for correct recruitment of adherens junction components and zonula occludens formation. Exp. Cell Res. 312, 1637–1650 (2006).
Smutny, M. et al. Multicomponent analysis of junctional movements regulatedby myosin II isoforms at the epithelial zonula adherens. PLoS One 6, e22458 (2011).
Kasza, K. E. & Zallen, J. A. Dynamics and regulation of contractile actin-myosin networks in morphogenesis. Curr. Opin. Cell Biol. 23, 30–38 (2011).
Monier, B., Pelissier-Monier, A., Brand, A. H. & Sanson, B. An actomyosin-based barrier inhibits cell mixing at compartmental boundaries in Drosophila embryos. Nat. Cell Biol. 12, 60–65 (2009).
Fernandez-Gonzalez, R., Simoes Sde, M., Roper, J. C., Eaton, S. & Zallen, J. A. Myosin II dynamics are regulated by tension in intercalating cells. Dev. Cell 17, 736–743 (2009).
Mishima, M., Kaitna, S. & Glotzer, M. Central spindle assembly and cytokinesis require a kinesin-like protein/RhoGAP complex with microtubule bundling activity. Dev. Cell 2, 41–54 (2002).
Hirose, K., Kawashima, T., Iwamoto, I., Nosaka, T. & Kitamura, T. MgcRacGAP is involved in cytokinesis through associating with mitotic spindle and midbody. J. Biol. Chem. 276, 5821–5828 (2001).
Mikawa, M., Su, L. & Parsons, S. J. Opposing roles of p190RhoGAP and Ect2 RhoGEF in regulating cytokinesis. Cell Cycle 7, 2003–2012 (2008).
Wildenberg, G. A. et al. p120-catenin and p190RhoGAP regulate cell–celladhesion by coordinating antagonism between Rac and Rho. Cell 127, 1027–1039 (2006).
Noren, N. K., Arthur, W. T. & Burridge, K. Cadherin engagement inhibits RhoA via p190RhoGAP. J. Biol. Chem. 278, 13615–13618 (2003).
Manchinelly, S. A. et al. Mitotic down-regulation of p190RhoGAP is required for the successful completion of cytokinesis. J. Biol. Chem. 285, 26923–26932 (2010).
Su, L., Pertz, O., Mikawa, M., Hahn, K. & Parsons, S. J. p190RhoGAP negatively regulates Rho activity at the cleavage furrow of mitotic cells. Exp. Cell Res. 315, 1347–1359 (2009).
Bustos, R. I., Forget, M. A., Settleman, J. E. & Hansen, S. H. Coordination of Rho and Rac GTPase function via p190B RhoGAP. Curr. Biol. 18, 1606–1611 (2008).
Burbelo, P. D. et al. p190-B, a new member of the Rho GAP family, and Rho are induced to cluster after integrin cross-linking. J. Biol. Chem. 270, 30919–30926 (1995).
Scott, J. A. & Yap, A. S. Cinderella no longer: alpha-catenin steps out of cadherin’s shadow. J. Cell Sci. 119, 4599–4605 (2006).
Lien, W. H., Klezovitch, O. & Vasioukhin, V. Cadherin-catenin proteins in vertebrate development. Curr. Opin. Cell Biol. 18, 499–506 (2006).
Terry, S. J. et al. Spatially restricted activation of RhoA signalling at epithelial junctions by p114RhoGEF drives junction formation and morphogenesis. Nat. Cell Biol. 13, 159–166 (2011).
Saint, R. & Somers, W. G. Animal cell division: a fellowship of the double ring? J. Cell Sci. 116, 4277–4281 (2003).
Bellett, G. et al. Microtubule plus-end and minus-end capture at adherens junctions is involved in the assembly of apico-basal arrays in polarised epithelial cells. Cell Motil. Cytoskeleton 66, 893–908 (2009).
Stehbens, S. J., Akhmanova, A. & Yap, A. S. Microtubules and cadherins: a neglected partnership. Front. Biosci. 14, 3159–3167 (2009).
Akhmanova, A., Stehbens, S. J. & Yap, A. S. Touch, grasp, deliver and control: functional cross-talk between microtubules and cell adhesions. Traffic 10, 268–274 (2009).
Rodriguez, O. C. et al. Conserved microtubule-actin interactions in cell movement and morphogenesis. Nat. Cell Biol. 5, 599–609 (2003).
Wolfe, B. A., Takaki, T., Petronczki, M. & Glotzer, M. Polo-like kinase 1 directs assembly of the HsCyk-4 RhoGAP/Ect2 RhoGEF complex to initiate cleavage furrow formation. PLoS Biol. 7, e1000110 (2009).
Ridley, A. J., Paterson, H. F., Johnston, C. L., Diekmann, D. & Hall, A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell 70, 401–410 (1992).
Itoh, R. E. et al. Activation of rac and cdc42 video imaged by fluorescent resonance energy transfer-based single-molecule probes in the membrane of living cells. Mol. Cell. Biol. 22, 6582–6591 (2002).
Grigoriev, I. et al. STIM1 is a MT-plus-end-tracking protein involved in remodelling of the ER. Curr. Biol. 18, 177–182 (2008).
Rubinson, D. A. et al. A lentivirus-based system to functionally silence genes in primary mammalian cells, stem cells and transgenic mice by RNA interference. Nat. Genet. 33, 401–406 (2003).
Vitriol, E. A., Uetrecht, A. C., Shen, F., Jacobson, K. & Bear, J. E. Enhanced EGFP-chromophore-assisted laser inactivation using deficient cells rescued with functional EGFP-fusion proteins. Proc. Natl Acad. Sci. USA 104, 6702–6707 (2007).
Reynolds, A. et al. Rational siRNA design for RNA interference. Nat. Biotechnol. 22, 326–330 (2004).
Verma, S. et al. Arp2/3 activity is necessary for efficient formation of E-cadherin adhesive contacts. J. Biol. Chem. 279, 34062–34070 (2004).
Acknowledgements
We thank our laboratory colleagues for their support and advice, all our colleagues who provided gifts of reagents, and R. Saint who first suggested we think about ECT2. This work was financially supported by the Human Frontiers Science Program, the National Health and Medical Research Council of Australia, Australian Research Council, and the Oncology Children’s Foundation. Confocal microscopy was performed at the IMB/ACRF Cancer Biology Imaging Facility, established with the generous support of the Australian Cancer Research Foundation.
Author information
Authors and Affiliations
Contributions
A.R., S.J.S., A.A. and A.S.Y. conceived the project, A.R., G.A.G., R.P., S.V. and N.H.B. conducted experiments, E.M.K. and K.J. generated reagents, A.R., G.A.G. and A.S.Y. analysed the data and A.R. and A.S.Y. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1840 kb)
Supplementary Table 1
Supplementary Information (XLS 35 kb)
Supplementary Table 2
Supplementary Information (XLS 40 kb)
Supplementary Table 3
Supplementary Information (XLS 41 kb)
Rights and permissions
About this article
Cite this article
Ratheesh, A., Gomez, G., Priya, R. et al. Centralspindlin and α-catenin regulate Rho signalling at the epithelial zonula adherens. Nat Cell Biol 14, 818–828 (2012). https://doi.org/10.1038/ncb2532
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2532
This article is cited by
-
Patterning of the cell cortex by Rho GTPases
Nature Reviews Molecular Cell Biology (2024)
-
Adherens junctions as molecular regulators of emergent tissue mechanics
Nature Reviews Molecular Cell Biology (2023)
-
α2β1 integrins spatially restrict Cdc42 activity to stabilise adherens junctions
BMC Biology (2021)
-
Collectively stabilizing and orienting posterior migratory forces disperses cell clusters in vivo
Nature Communications (2020)
-
Polarity signaling ensures epidermal homeostasis by coupling cellular mechanics and genomic integrity
Nature Communications (2019)