Abstract
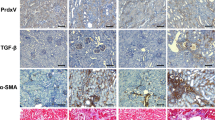
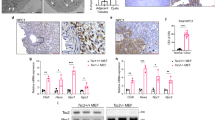
Mammalian target of rapamycin complex 2 (mTORC2) phosphorylates AGC protein kinases including protein kinase C (PKC) and regulates cellular functions such as cell migration. However, its regulation remains poorly understood. Here we show that lysophosphatidic acid (LPA) induces two phases of PKC-δ hydrophobic motif phosphorylation. The late phase is mediated by Gα12, which specifically activates ARAF, leading to upregulation of the RFFL E3 ubiquitin ligase and subsequent ubiquitylation and degradation of the PRR5L subunit of mTORC2. Destabilization of PRR5L, a suppressor of mTORC2-mediated hydrophobic motif phosphorylation of PKC-δ, but not AKT, results in PKC-δ hydrophobic motif phosphorylation and activation. This Gα12-mediated signalling pathway for mTORC2 regulation is critically important for fibroblast migration and pulmonary fibrosis development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Accession codes
References
Polak, P. & Hall, M. N. mTOR and the control of whole body metabolism. Curr. Opin. Cell Biol. 21, 209–218 (2009).
Wullschleger, S., Loewith, R. & Hall, M. N. TOR signaling in growth and metabolism. Cell 124, 471–484 (2006).
Zoncu, R., Efeyan, A. & Sabatini, D. M. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat. Rev. Mol. Cell Biol. 12, 21–35 (2011).
Foster, K. G. & Fingar, D. C. Mammalian target of rapamycin (mTOR): conducting the cellular signaling symphony. J. Biol. Chem. 285, 14071–14077 (2010).
Huang, J. & Manning, B. D. A complex interplay between Akt, TSC2 and the two mTOR complexes. Biochem. Soc. Trans. 37, 217–222 (2009).
Fonseca, B. D., Smith, E. M., Lee, V. H., MacKintosh, C. & Proud, C. G. PRAS40 is a target for mammalian target of rapamycin complex 1 and is required for signaling downstream of this complex. J. Biol. Chem. 282, 24514–24524 (2007).
Hara, K. et al. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell 110, 177–189 (2002).
Kim, D. H. et al. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell 110, 163–175 (2002).
Kim, D. H. et al. GβL, a positive regulator of the rapamycin-sensitive pathway required for the nutrient-sensitive interaction between raptor and mTOR. Mol. Cell 11, 895–904 (2003).
Kovacina, K. S. et al. Identification of a proline-rich Akt substrate as a 14-3-3 binding partner. J. Biol. Chem. 278, 10189–10194 (2003).
Jacinto, E. et al. SIN1/MIP1 maintains rictor–mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell 127, 125–137 (2006).
Pearce, L. R. et al. Identification of Protor as a novel Rictor-binding component of mTOR complex-2. Biochem J 405, 513–522 (2007).
Sarbassov, D. D. et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr. Biol. 14, 1296–1302 (2004).
Woo, S. Y. et al. PRR5, a novel component of mTOR complex 2, regulates platelet-derived growth factor receptor β expression and signaling. J. Biol. Chem. 282, 25604–25612 (2007).
Yang, Q., Inoki, K., Ikenoue, T. & Guan, K. L. Identification of Sin1 as an essential TORC2 component required for complex formation and kinase activity. Genes Dev. 20, 2820–2832 (2006).
Jacinto, E. & Lorberg, A. TOR regulation of AGC kinases in yeast and mammals. Biochem. J. 410, 19–37 (2008).
Mamane, Y., Petroulakis, E., LeBacquer, O. & Sonenberg, N. mTOR, translation initiation and cancer. Oncogene 25, 6416–6422 (2006).
Tremblay, F. & Marette, A. Amino acid and insulin signaling via the mTOR/p70 S6 kinase pathway. A negative feedback mechanism leading to insulin resistance in skeletal muscle cells. J. Biol. Chem. 276, 38052–38060 (2001).
Um, S. H. et al. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature 431, 200–205 (2004).
Facchinetti, V. et al. The mammalian target of rapamycin complex 2 controls folding and stability of Akt and protein kinase C. EMBO J. 27, 1932–1943 (2008).
Garcia-Martinez, J. M. & Alessi, D. R. mTOR complex 2 (mTORC2) controls hydrophobic motif phosphorylation and activation of serum- and glucocorticoid-induced protein kinase 1 (SGK1). Biochem. J. 416, 375–385 (2008).
Ikenoue, T., Inoki, K., Yang, Q., Zhou, X. & Guan, K. L. Essential function of TORC2 in PKC and Akt turn motif phosphorylation, maturation and signalling. EMBO J. 27, 1919–1931 (2008).
Loewith, R. et al. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol. Cell 10, 457–468 (2002).
Sarbassov, D. D., Guertin, D. A., Ali, S. M. & Sabatini, D. M. Phosphorylation and regulation of Akt/PKB by the rictor–mTOR complex. Science 307, 1098–1101 (2005).
Jacinto, E. et al. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat. Cell Biol. 6, 1122–1128 (2004).
Schmidt, A., Kunz, J. & Hall, M. N. TOR2 is required for organization of the actin cytoskeleton in yeast. Proc. Natl Acad. Sci. USA 93, 13780–13785 (1996).
Liu, L., Das, S., Losert, W. & Parent, C. A. mTORC2 regulates neutrophil chemotaxis in a cAMP- and RhoA-dependent fashion. Dev. cell 19, 845–857 (2010).
Copp, J., Manning, G. & Hunter, T. TORC-specific phosphorylation of mammalian target of rapamycin (mTOR): phospho-Ser2481 is a marker for intact mTOR signaling complex 2. Cancer Res. 69, 1821–1827 (2009).
Soliman, G. A. et al. mTOR Ser-2481 autophosphorylation monitors mTORC-specific catalytic activity and clarifies rapamycin mechanism of action. J. Biol. Chem. 285, 7866–7879 (2010).
Gan, X., Wang, J., Su, B. & Wu, D. Evidence for direct activation of mTORC2 kinase activity by phosphatidylinositol 3,4,5-trisphosphate. J. Biol. Chem. 286, 10998–11002 (2011).
Zinzalla, V., Stracka, D., Oppliger, W. & Hall, M. N. Activation of mTORC2 by association with the ribosome. Cell 144, 757–768 (2011).
Oh, W. J. et al. mTORC2 can associate with ribosomes to promote cotranslational phosphorylation and stability of nascent Akt polypeptide. EMBO J. 29, 3939–3951 (2010).
Worzfeld, T., Wettschureck, N. & Offermanns, S. G(12)/G(13)-mediated signalling in mammalian physiology and disease. Trends Pharmacol. Sci. 29, 582–589 (2008).
Riobo, N. A. & Manning, D. R. Receptors coupled to heterotrimeric G proteins of the G12 family. Trends Pharmacol. Sci. 26, 146–154 (2005).
Choi, J. W. et al. LPA receptors: subtypes and biological actions. Annu. Rev. Pharmacol. Toxicol. 50, 157–186 (2010).
Karagiosis, S. A., Chrisler, W. B., Bollinger, N. & Karin, N. J. Lysophosphatidic acid-induced ERK activation and chemotaxis in MC3T3-E1 preosteoblastsare independent of EGF receptor transactivation. J. Cell Physiol. 219, 716–723 (2009).
Herroeder, S. et al. Guanine nucleotide-binding proteins of the G12 family shape immune functions by controlling CD4+ T cell adhesiveness and motility. Immunity 30, 708–720 (2009).
Gong, H. et al. G protein subunit Gα13 binds to integrin αIIbβ3 and mediates integrin ‘outside-in’ signaling. Science 327, 340–343 (2010).
Moers, A. et al. G13 is an essential mediator of platelet activation in hemostasis and thrombosis. Nat. Med. 9, 1418–1422 (2003).
Shan, D. et al. The G protein Gα13 is required for growth factor-induced cell migration. Dev. cell 10, 707–718 (2006).
Radhika, V., Hee Ha, J., Jayaraman, M., Tsim, S. T. & Dhanasekaran, N. Mitogenic signaling by lysophosphatidic acid (LPA) involves Gα12 . Oncogene 24, 4597–4603 (2005).
Ki, S. H., Choi, M. J., Lee, C. H. & Kim, S. G. Gα12 specifically regulates COX-2 induction by sphingosine 1-phosphate. Role for JNK-dependent PKC-αion and degradation of IκBα. J. Biol. Chem. 282, 1938–1947 (2007).
Won, H. Y., Min, H. J., Lee, W. H., Kim, S. G. & Hwang, E. S. Gα12 is critical for TCR-induced IL-2 production and differentiation of T helper 2 and T helper 17 cells. Biochem. Biophys. Res. Com. 394, 811–816 (2010).
Tager, A. M. et al. The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat. Med. 14, 45–54 (2008).
Neubert, K. et al. The proteasome inhibitor bortezomib depletes plasma cells and protects mice with lupus-like disease from nephritis. Nat. Med. 14, 748–755 (2008).
Janes, M. R. et al. Effective and selective targeting of leukemia cells using a TORC1/2 kinase inhibitor. Nat. Med. 16, 205–213 (2010).
Coumailleau, F. et al. Over-expression of Rififylin, a new RING finger and FYVE-like domain-containing protein, inhibits recycling from the endocytic recycling compartment. Mol. Biol. Cell 15, 4444–4456 (2004).
McDonald, E. R. 3rd & El-Deiry, W. S. Suppression of caspase-8- and -10-associated RING proteins results in sensitization to death ligands and inhibition of tumor cell growth. Proc. Natl Acad. Sci. USA 101, 6170–6175 (2004).
Yang, W. et al. CARPs are ubiquitin ligases that promote MDM2-independent p53 and phospho-p53ser20 degradation. J. Biol. Chem. 282, 3273–3281 (2007).
Liao, W. et al. CARP-2 is an endosome-associated ubiquitin ligase for RIP and regulates TNF-induced NF-κB activation. Curr. Biol. CB 18, 641–649 (2008).
Woo, S. Y. et al. PRR5, a novel component of mTOR complex 2, regulates platelet-derived growth factor receptor β expression and signaling. J. Biol. Chem. 282, 25604–25612 (2007).
Thedieck, K. et al. PRAS40 and PRR5-like protein are new mTOR interactors that regulate apoptosis. PLoS One 2, e1217 (2007).
Pearce, L. R. et al. Identification of Protor as a novel Rictor-binding component of mTOR complex-2. Biochem. J. 405, 513–522 (2007).
Pearce, L. R., Sommer, E. M., Sakamoto, K., Wullschleger, S. & Alessi, D. R. Protor-1 is required for efficient mTORC2-mediated activation of SGK1 in the kidney. Biochem. J. 436, 169–179 (2011).
Bai, X. et al. Protein kinase C{δ} deficiency accelerates neointimal lesions of mouse injured artery involving delayed reendothelialization and vasohibin-1 accumulation. Arterioscler. Thromb. Vasc. Biol. 30, 2467–2474 (2010).
Zhao, C. T. et al. PKCδ regulates cortical radial migration by stabilizing the Cdk5 activator p35. Proc. Natl Acad. Sci. USA 106, 21353–21358 (2009).
Liu, B. et al. Protein kinase C-δ regulates migration and proliferation of vascular smooth muscle cells through the extracellular signal-regulated kinase 1/2. J. Vasc. Surg. 45, 160–168 (2007).
Chou, W. H. et al. Neutrophil protein kinase C δ as a mediator of stroke-reperfusion injury. J. Clin. Invest. 114, 49–56 (2004).
Zhang, Y. et al. Different roles of G protein subunits β1 and β2 in neutrophil function revealed by gene expression silencing in primary mouse neutrophils. J. Biol. Chem. 285, 24805–24814 (2010).
Hart, M. J. et al. Direct stimulation of the guanine nucleotide exchange activity of p115 RhoGEF by Gα13 . Science 280, 2112–2114 (1998).
Kozasa, T. et al. p115 RhoGEF, a GTPase activating protein for Gα12 and Gα13 . Science 280, 2109–2111 (1998).
Gu, J. L., Muller, S., Mancino, V., Offermanns, S. & Simon, M. I. Interaction of Gα(12) with Gα(13) and Gα(q) signaling pathways. Proc. Natl Acad. Sci. USA 99, 9352–9357 (2002).
Tager, A. M. et al. Inhibition of pulmonary fibrosis by the chemokine IP-10/CXCL10. Am. J. Respir. Cell Mol. Biol. 31, 395–404 (2004).
Acknowledgements
We thank B. Su for discussion and the anti-MAPKAP1 antibody, and M. Orsulak for technical help. This work is supported by National Institutes of Health grants (HL070694, HL080706 and CA139395 to D.W. and GM61454 and GM074001 to T.K.) and an Ellison Medical Foundation grant (AG-SS-2190-08 to M.I.S.).
Author information
Authors and Affiliations
Contributions
X.G. conceived the initial idea, planned and carried out some of the experiments, analysed the data and participated in manuscript preparation. J.W. and C.W. carried out some of the experiments. E.S., T.K., S.S. and D.A. provided experimental materials. S.O. and M.I.S. provided research materials and comments and helped in manuscript preparation. D.W. conceived the initial idea, planed the experiments, analysed the data and prepared the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 2714 kb)
Supplementary Table 1
Supplementary Information (XLS 36 kb)
Rights and permissions
About this article
Cite this article
Gan, X., Wang, J., Wang, C. et al. PRR5L degradation promotes mTORC2-mediated PKC-δ phosphorylation and cell migration downstream of Gα12. Nat Cell Biol 14, 686–696 (2012). https://doi.org/10.1038/ncb2507
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2507
This article is cited by
-
mTORC2: a multifaceted regulator of autophagy
Cell Communication and Signaling (2023)
-
Characterization of genome-wide STR variation in 6487 human genomes
Nature Communications (2023)
-
ETS-1-activated LINC01016 over-expression promotes tumor progression via suppression of RFFL-mediated DHX9 ubiquitination degradation in breast cancers
Cell Death & Disease (2023)
-
Somatic ARAF mutations in pediatric Langerhans cell histiocytosis: clinicopathologic, genetic and functional profiling
Clinical and Experimental Medicine (2023)
-
Targeting chemotherapy resistance in mesenchymal triple-negative breast cancer: a phase II trial of neoadjuvant angiogenic and mTOR inhibition with chemotherapy
Investigational New Drugs (2023)