Abstract
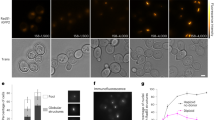
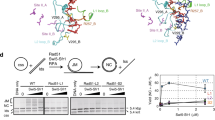
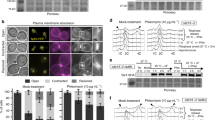
Chromatin mobility is thought to facilitate homology search during homologous recombination and to shift damage either towards or away from specialized repair compartments. However, unconstrained mobility of double-strand breaks could also promote deleterious chromosomal translocations. Here we use live time-lapse fluorescence microscopy to track the mobility of damaged DNA in budding yeast. We found that a Rad52–YFP focus formed at an irreparable double-strand break moves in a larger subnuclear volume than the undamaged locus. In contrast, Rad52–YFP bound at damage arising from a protein–DNA adduct shows no increase in movement. Mutant analysis shows that enhanced double-strand-break mobility requires Rad51, the ATPase activity of Rad54, the ATR homologue Mec1 and the DNA-damage-response mediator Rad9. Consistent with a role for movement in the homology-search step of homologous recombination, we show that recombination intermediates take longer to form in cells lacking Rad9.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Gehlen, L., Gasser, S. M. & Dion, V. How broken DNA finds a template for repair: a computational approach. Prog. Theor. Phys. Suppl. 191, 20–29 (2011).
Savage, J. R. Insight into sites. Mutat. Res. 366, 81–95 (1996).
Dimitrova, N., Chen, Y. C., Spector, D. L. & de Lange, T. 53BP1 promotes non-homologous end joining of telomeres by increasing chromatin mobility. Nature 456, 524–528 (2008).
Noon, A. T. & Goodarzi, A. A. 53BP1-mediated DNA double strand break repair: insert bad pun here. DNA Repair (Amst) 10, 1071–1076 (2011).
Aten, J. A. et al. Dynamics of DNA double-strand breaks revealed by clustering of damaged chromosome domains. Science 303, 92–95 (2004).
Lisby, M., Mortensen, U. H. & Rothstein, R. Colocalization of multiple DNA double-strand breaks at a single Rad52 repair centre. Nat. Cell Biol. 5, 572–577 (2003).
Jakob, B., Splinter, J., Durante, M. & Taucher-Scholz, G. Live cell microscopy analysis of radiation-induced DNA double-strand break motion. Proc. Natl Acad. Sci. USA 106, 3172–3177 (2009).
Jakob, B., Splinter, J. & Taucher-Scholz, G. Positional stability of damaged chromatin domains along radiation tracks in mammalian cells. Radiat. Res. 171, 405–418 (2009).
Kruhlak, M. J. et al. Changes in chromatin structure and mobility in living cells at sites of DNA double-strand breaks. J. Cell Biol. 172, 823–834 (2006).
Soutoglou, E. et al. Positional stability of single double-strand breaks in mammalian cells. Nat. Cell Biol. 9, 675–682 (2007).
Nelms, B. E., Maser, R. S., MacKay, J. F., Lagally, M. G. & Petrini, J. H. In situ visualization of DNA double-strand break repair in human fibroblasts. Science 280, 590–592 (1998).
Gartenberg, M. R., Neumann, F. R., Laroche, T., Blaszczyk, M. & Gasser, S. M. Sir-mediated repression can occur independently of chromosomal and subnuclear contexts. Cell 119, 955–967 (2004).
Meister, P., Gehlen, L. R., Varela, E., Kalck, V. & Gasser, S. M. Visualizing yeast chromosomes and nuclear architecture. Methods Enzymol. 470, 535–567 (2010).
Neumann, F. R. et al. INO80 promotes chromatin movement and functionally impacts homologous recombination. Genes Dev. 26, 369–383 (2012).
Straight, A. F., Belmont, A. S., Robinett, C. C. & Murray, A. W. GFP tagging of budding yeast chromosomes reveals that protein–protein interactions can mediate sister chromatid cohesion. Curr. Biol. 6, 1599–1608 (1996).
Lisby, M., Barlow, J. H., Burgess, R. C. & Rothstein, R. Choreography of the DNA damage response: spatiotemporal relationships among checkpoint and repair proteins. Cell 118, 699–713 (2004).
Heun, P., Laroche, T., Shimada, K., Furrer, P. & Gasser, S. M. Chromosome dynamics in the yeast interphase nucleus. Science 294, 2181–2186 (2001).
Povirk, L. F. DNA damage and mutagenesis by radiomimetic DNA-cleaving agents: bleomycin, neocarzinostatin and other enediynes. Mutat. Res. 355, 71–89 (1996).
Mazin, A. V., Mazina, O. M., Bugreev, D. V. & Rossi, M. J. Rad54, the motor of homologous recombination. DNA Repair (Amst) 9, 286–302 (2010).
Clever, B., Schmuckli-Maurer, J., Sigrist, M., Glassner, B. J. & Heyer, W. D. Specific negative effects resulting from elevated levels of the recombinational repair protein Rad54p in Saccharomyces cerevisiae. Yeast 15, 721–740 (1999).
Nielsen, I. et al. A Flp-nick system to study repair of a single protein-bound nick in vivo. Nat. Methods 6, 753–757 (2009).
Mochan, T. A., Venere, M., DiTullio, R. A. Jr & Halazonetis, T. D. 53BP1, an activator of ATM in response to DNA damage. DNA Repair (Amst) 3, 945–952 (2004).
Nyberg, K. A., Michelson, R. J., Putnam, C. W. & Weinert, T. A. Toward maintaining the genome: DNA damage and replication checkpoints. Annu. Rev. Genet. 36, 617–656 (2002).
Stucki, M. & Jackson, S.P. MDC1/NFBD1: a key regulator of the DNA damage response in higher eukaryotes. DNA Repair (Amst) 3, 953–957 (2004).
Vialard, J. E., Gilbert, C. S., Green, C. M. & Lowndes, N. F. The budding yeast Rad9 checkpoint protein is subjected to Mec1/Tel1-dependent hyperphosphorylation and interacts with Rad53 after DNA damage. EMBO J. 17, 5679–5688 (1998).
Sweeney, F. D. et al. Saccharomyces cerevisiae Rad9 acts as a Mec1 adaptor to allow Rad53 activation. Curr. Biol. 15, 1364–1375 (2005).
Usui, T., Foster, S. S. & Petrini, J. H. Maintenance of the DNA-damage checkpoint requires DNA-damage-induced mediator protein oligomerization. Mol. Cell 33, 147–159 (2009).
Conde, F. et al. The Dot1 histone methyltransferase and the Rad9 checkpoint adaptor contribute to cohesin-dependent double-strand break repair by sister chromatid recombination in Saccharomyces cerevisiae. Genetics 182, 437–446 (2009).
Zhao, X., Muller, E. G. & Rothstein, R. A suppressor of two essential checkpoint genes identifies a novel protein that negatively affects dNTP pools. Mol Cell 2, 329–340 (1998).
Barlow, J. H. & Rothstein, R. Rad52 recruitment is DNA replication independent and regulated by Cdc28 and the Mec1 kinase. EMBO J. 28, 1121–1130 (2009).
White, C. I. & Haber, J. E. Intermediates of recombination during mating type switching in Saccharomyces cerevisiae. EMBO J. 9, 663–673 (1990).
Frank-Vaillant, M. & Marcand, S. Transient stability of DNA ends allows nonhomologous end joining to precede homologous recombination. Mol. Cell 10, 1189–1199 (2002).
Nagai, S. et al. Functional targeting of DNA damage to a nuclear pore-associated SUMO-dependent ubiquitin ligase. Science 322, 597–602 (2008).
Pellicioli, A., Lee, S. E., Lucca, C., Foiani, M. & Haber, J. E. Regulation of Saccharomyces Rad53 checkpoint kinase during adaptation from DNA damage-induced G2/M arrest. Mol. Cell 7, 293–300 (2001).
Miné-Hattab, J. & Rothstein, R. Increased chromosome mobility: a model for homology search during homologous recombination. Nat. Cell Biol.http://dx.doi.org/10.1038/ncb2472 (2012).
Dubrana, K., van Attikum, H., Hediger, F. & Gasser, S. M. The processing of double-strand breaks and binding of single-strand-binding proteins RPA and Rad51 modulate the formation of ATR-kinase foci in yeast. J. Cell Sci. 120, 4209–4220 (2007).
Zou, L. & Elledge, S. J. Sensing DNA damage through ATRIP recognition of RPA–ssDNA complexes. Science 300, 1542–1548 (2003).
Bennett, C. B., Lewis, A. L., Baldwin, K. K. & Resnick, M. A. Lethality induced by a single site-specific double-strand break in a dispensable yeast plasmid. Proc. Natl Acad. Sci. USA 90, 5613–5617 (1993).
Chiolo, I. et al. Double-strand breaks in heterochromatin move outside of a dynamic HP1a domain to complete recombinational repair. Cell 144, 732–744 (2011).
Torres-Rosell, J. et al. The Smc5–Smc6 complex and SUMO modification of Rad52 regulates recombinational repair at the ribosomal gene locus. Nat. Cell Biol. 9, 923–931 (2007).
Ponti, A., Gulati, A., Bäcker, V. & Schwarb, P. Huygens Remote Manager: a web interface for high-volume batch deconvolution. Imaging Microscopy 9, 57–58 (2007).
Sage, D., Neumann, F. R., Hediger, F., Gasser, S. M. & Unser, M. Automatic tracking of individual fluorescence particles: application to the study of chromosome dynamics. IEEE Trans. Image Process 14, 1372–1383 (2005).
Kim, Y. H. et al. Chromosome XII context is important for rDNA function in yeast. Nucleic Acids Res. 34, 2914–2924 (2006).
Acknowledgements
We thank R. Schmid for imaging assistance, H. van Attikum for cloning assistance and L. Bjergbaek for the Flp-nick strain and sharing unpublished data. We thank W. Heyer for the Rad54 mutant construct, J. E. Haber for JKM154 and R. Rothstein and J. Mine-Hattab for sharing unpublished reagents and results. We thank B. Pike, K. Shimada, A. Gonzalez, N. Hustedt, M. Oppikofer, A. Seeber and F. Hamaratoglu for reading the manuscript and the Friedrich Miescher Institute Facility for Advanced Imaging and Microscopy for technical help. V.D. is supported in part by a postdoctoral award from the Terry Fox Foundation (award no. 19759) and work in S.M.G.’s laboratory is supported by the Novartis Research Foundation and the Swiss National Science Foundation National Centre of Competence in Research ‘Frontiers in genetics’ programme.
Author information
Authors and Affiliations
Contributions
V.D. and S.M.G. designed the experiments, analysed the results and wrote the paper. V.D. and V.K. carried out the experiments. C.H. provided the cutting-efficiency data. B.D.T. tested and optimized the I-SceI cleavage.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 431 kb)
Rights and permissions
About this article
Cite this article
Dion, V., Kalck, V., Horigome, C. et al. Increased mobility of double-strand breaks requires Mec1, Rad9 and the homologous recombination machinery. Nat Cell Biol 14, 502–509 (2012). https://doi.org/10.1038/ncb2465
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2465
This article is cited by
-
Distinct characteristics of the DNA damage response in mammalian oocytes
Experimental & Molecular Medicine (2024)
-
In vivo tracking of functionally tagged Rad51 unveils a robust strategy of homology search
Nature Structural & Molecular Biology (2023)
-
Multiscale reorganization of the genome following DNA damage facilitates chromosome translocations via nuclear actin polymerization
Nature Structural & Molecular Biology (2023)
-
MasterPATH: network analysis of functional genomics screening data
BMC Genomics (2020)
-
Fission yeast condensin contributes to interphase chromatin organization and prevents transcription-coupled DNA damage
Genome Biology (2020)