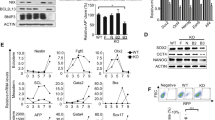
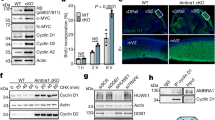
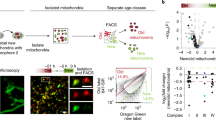
Abstract
The midbody is a singular organelle formed between daughter cells during cytokinesis and required for their final separation. Midbodies persist in cells long after division as midbody derivatives (MBds), but their fate is unclear. Here we show that MBds are inherited asymmetrically by the daughter cell with the older centrosome. They selectively accumulate in stem cells, induced pluripotent stem cells and potential cancer ‘stem cells’ in vivo and in vitro. MBd loss accompanies stem-cell differentiation, and involves autophagic degradation mediated by binding of the autophagic receptor NBR1 to the midbody protein CEP55. Differentiating cells and normal dividing cells do not accumulate MBds and possess high autophagic activity. Stem cells and cancer cells accumulate MBds by evading autophagosome encapsulation and exhibit low autophagic activity. MBd enrichment enhances reprogramming to induced pluripotent stem cells and increases the in vitro tumorigenicity of cancer cells. These results indicate unexpected roles for MBds in stem cells and cancer ‘stem cells’.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Change history
15 November 2011
In the version of this article initially published online, the first sentence in the Acknowledgements section was incorrect. This error has been corrected in the HTML and PDF versions of the article.
References
Eggert, U. S., Mitchison, T. J. & Field, C. M. Animal cytokinesis: from parts list to mechanisms. Annu. Rev. Biochem. 75, 543–66 (2006).
Neumüller, R. A. & Knoblich, J. A. Dividing cellular asymmetry: asymmetric cell division and its implications for stem cells and cancer. Genes Dev. 23, 2675–2699 (2009).
Doxsey, S., McCollum, D. & Theurkauf, W. Centrosomes in cellular regulation. Annu. Rev. Cell Dev. Biol. 21, 411–434 (2005).
Yamashita, Y. M., Mahowald, A. P., Perlin, J. R. & Fuller, M. T. Asymmetric inheritance of mother versus daughter centrosome in stem cell division. Science 315, 518–521 (2007).
Wang, X. et al. Asymmetric centrosome inheritance maintains neural progenitors in the neocortex. Nature 461, 947–955 (2009).
Yamashita, Y. M., Jones, D. L. & Fuller, M. T. Orientation of asymmetric stem cell division by the APC tumor suppressor and centrosome. Science 301, 1547–1550 (2003).
Barr, F. A. & Gruneberg, U. Cytokinesis: placing and making the final cut. Cell 131, 847–860 (2007).
Mullins, J. M. & Biesele, J. J. Terminal phase of cytokinesis in D-98s cells. J. Cell Biol. 73, 672–684 (1977).
Gromley, et al. Centriolin anchoring of exocyst and SNARE complexes at the midbody is required for secretory-vesicle-mediated abscission. Cell 123, 75–87 (2005).
Steigemann, P. et al. Aurora B-mediated abscission checkpoint protects against tetraploidization. Cell 136, 473–484 (2009).
Goss, J. W. & Toomre, D. K. Both daughter cells traffic and exocytose membrane at the cleavage furrow during mammalian cytokinesis. J. Cell Biol. 181, 1047–1054 (2008).
Pohl, C. & Jentsch, S. Midbody ring disposal by autophagy is a post-abscission event of cytokinesis. Nat. Cell Biol. 11, 65–70 (2009).
Marzesco, A. M. et al. Release of extracellular membrane particles carrying the stem cell marker prominin-1 (CD133) from neural progenitors and other epithelial cells. J. Cell Sci. 118, 2849–2858 (2005).
Dubreuil, V. et al. Midbody and primary cilium of neural progenitors release extracellular membrane particles enriched in the stem cell marker prominin-1. J. Cell. Biol. 176, 483–495 (2007).
Mizushima, N., Levine, B., Cuervo, A. M. & Klionsky, D. J. Autophagy fights disease through cellular self-digestion. Nature 451, 1069–1075 (2008).
Mizushima, N. & Klionsky, D. Protein turnover via autophagy: implications for metabolism. Annu. Rev. Nutr. 27, 19–40 (2007).
Yorimitsu, T. & Klionsky, D. J. Eating the endoplasmic reticulum: quality control by autophagy. Trends Cell Biol. 17, 279–285 (2007).
Levine, B. & Kroemer, G. Autophagy in the pathogenesis of disease. Cell 132, 27–42 (2008).
Kuma, A. et al. The role of autophagy during the early neonatal starvation period. Nature 432, 1032–1036 (2004).
Fimia, G. M. et al. Ambra1 regulates autophagy and development of the nervous system. Nature 447, 1121–1125 (2007).
Tsukamoto, S. et al. Autophagy is essential for preimplantation development of mouse embryos. Science 321, 117–120 (2008).
Cecconi, F. & Levine, B. The role of autophagy in mammalian development: cell makeover rather than cell death. Dev. Cell 15, 344–357 (2008).
Hara, T. et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature 441, 885–889 (2006).
Komatsu, M. et al. Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J. Cell Biol. 169, 425–434 (2005).
Rujano, M. A. et al. Polarised asymmetric inheritance of accumulated protein damage in higher eukaryotes. PLoS Biol. 4, 2325–2335 (2006).
Johnston, J. A., Illing, M. E. & Kopito, R. R. Cytoplasmic dynein/dynactin mediates the assembly of aggresomes. Cell. Motil. Cytoskeleton 53, 26–38 (2002).
Anderson, C. T. & Stearns, T. Centriole age underlies asynchronous primary cilium growth in mammalian cells. Curr. Biol. 19, 1498–1502 (2009).
Piel, M., Meyer, P., Khodjakov, A., Rieder, C. L. & Bornens, M. The respective contributions of the mother and daughter centrioles to centrosome activity and behavior in vertebrate cells. J. Cell Biol. 149, 317–330 (2000).
Oatley, J. M. & Brinster, R. L. Regulation of spermatogonial stem cell self-renewal in mammals. Annu. Rev. Cell Dev. Biol. 24, 263–286 (2008).
Barroca, V. et al. Mouse differentiating spermatogonia can generate germinal stem cells in vivo. Nat. Cell Biol. 11, 190–196 (2009).
Bilgüvar, K. et al. Whole-exome sequencing identifies recessive WDR62 mutations in severe brain malformations. Nature 467, 207–210 (2010).
Morris, R. J. et al. Capturing and profiling adult hair follicle stem cells. Nat. Biotechnol. 22, 411–417 (2004).
Conboy, M. J., Cerletti, M., Wagers, A. J. & Conboy, I. M. Immuno-analysis and FACS sorting of adult muscle fiber-associated stem/precursor cells. Methods Mol. Biol. 621, 165–173 (2010).
Park, I. H. et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature 451, 141–146 (2008).
Chan, E. M. et al. Live cell imaging distinguishes bona fide human iPS cells from partially reprogrammed cells. Nat. Biotechnol. 27, 1033–1037 (2009).
Zwaka, T. P. & Thomson, J. A. Homologous recombination in human embryonic stem cells. Nat. Biotechnol. 21, 319–321 (2003).
Visvader, J. E. & Lindeman, G. J. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat. Rev. Cancer 8, 755–768 (2008).
O’Brien, C. A., Pollett, A., Gallinger, S. & Dick, J. E. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature 445, 106–110 (2007).
Pece, S. et al. Biological and molecular heterogeneity of breast cancers correlates with their cancer stem cell content. Cell 140, 62–73 (2010).
Pardal, R., Clarke, M. F. & Morrison, S. J. Applying the principles of stem-cell biology to cancer. Nat. Rev. Cancer 3, 895–902 (2003).
Salic, A. & Mitchison, T. J. A chemical method for fast and sensitive detection of DNA synthesis in vivo. Proc. Natl Acad. Sci. USA 105, 2415–2420 (2008).
Lorenz, H., Hailey, D. W. & Lippincott-Schwartz, J. Fluorescence protease protection of GFP chimeras to reveal protein topology and subcellular localization. Nat. Methods 3, 205–210 (2006).
Eskelinen, E. L., Tanaka, Y. & Saftig, P. At the acidic edge: emerging functions for lysosomal membrane proteins. Trends Cell Biol. 13, 137–145 (2003).
Klionsky, D. J. et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy 4, 151–175 (2008).
Liang, X. H. et al. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature 402, 672–676 (1999).
Sato, K. et al. Autophagy is activated in colorectal cancer cells and contributes to the tolerance to nutrient deprivation. Cancer Res. 67, 9677–9684 (2007).
Sarkar, S. et al. A rational mechanism for combination treatment of Huntington’s disease using lithium and rapamycin. Hum. Mol. Genet. 17, 170–178 (2008).
Sarkar, S. et al. Lithium induces autophagy by inhibiting inositol monophosphatase. J. Cell Biol. 170, 1101–1111 (2005).
Mizushima, N. & Yoshimori, T. How to interpret LC3 immunoblotting. Autophagy 3, 542–545 (2007).
Bjorkoy, G. et al. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J. Cell Biol. 171, 603–614 (2005).
Komatsu, M. et al. Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell 131, 1149–1163 (2007).
Pankiv, S. et al. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of of ubiquitinated protein aggregates by autophagy. J. Biol. Chem. 282, 24131–24145 (2007).
Kirkin, V. et al. A role for NBR1 in autophagosomal degradation of ubiquitinated substrates. Mol. Cell 27, 505–516 (2009).
Waters, S. et al. Interactions with LC3 and polyubiquitin chains link nbr1 to autophagic protein turnover. FEBS Lett. 583, 1846–1852 (2009).
Yu, J. et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 318, 1917–1920 (2007).
Engelmann, K., Shen, H. & Finn, O. J. MCF7 side population cells with characteristics of cancer stem/progenitor cells express the tumor antigen MUC1. Cancer Res. 68, 2419–2426 (2008).
Zhang, Y. et al. SEPA-1 mediates the specific recognition and degradation of P granule components by autophagy in C. elegans. Cell 136, 308–321 (2009).
Strome, S. Specification of the germ line. WormBook 28, 1–10 (2005).
Fuchs, E. The tortoise and the hair: slow-cycling cells in the stem cell race. Cell 137, 811–819 (2009).
Lamark, T. et al. Interaction codes within the family of mammalian Phox and Bem1p domain-containing proteins. J. Biol. Chem. 278, 34568–34581 (2003).
Majeski, A. E. & Dice, J. F. Mechanisms of chaperone-mediated autophagy. Int. J. Biochem. Cell Biol. 36, 2435–2444 (2004).
Kaushik, et al. Constitutive activation of chaperone-mediated autophagy in cells with impaired macroautophagy. Mol. Biol. Cell 19, 2179–2192 (2008).
Nedelsky, N. et al. Autophagy and the ubiquitin–proteasome system: collaborators in neuroprotection. Biochim. Biophys. Acta. 1782, 691–699 (2008).
Xu, P. & Davis, R. J. c-Jun NH2-terminal kinase is required for lineage-specific differentiation but not stem cell self-renewal. Mol. Cell Biol. 30, 1329–1340 (2010).
Greenbaum, M. P., Ma, L. & Matzuk, M. M. Conversion of midbodies into germ cell intercellular bridges. Dev. Biol. 305, 389–396 (2007).
Mitchison, T., Evans, L., Schulze, E. & Kirschner, M. Sites of microtubule assembly and disassembly in the mitotic spindle. Cell 45, 515–527 (1986).
Yu, L. et al. Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8. Science 304, 1500–1502 (2004).
Loewer, S. et al. Large intergenic non-coding RNA-RoR modulates reprogramming of human induced pluripotent stem cells. Nat. Genet. 42, 1113–1117 (2010).
Yu, J. et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science 324, 797–801 (2009).
Sachdev, S., Bu, Y. & Gelman, I. H. Paxillin-Y118 phosphorylation contributes to the control of Src-induced anchorage-independent growth by FAK and adhesion. BMC Cancer 12, 9–12 (2009).
Acknowledgements
We thank E. Baehrecke for critical reading of the manuscript, T. Schlaeger and colleagues for assistance with H1-OGN and associated cell lines, the University of Massachusetts Medical School (UMMS) Flow Facility for assistance with MBd enrichment, P. Furcinitti of UMMS Digital Light Microscopy Core Facility for assistance with imaging, the UMMS DERC Morphology Core for assistance with immunohistochemitry, D. Guertin and C. Sparks for assistance with SMP preparation, S. Lyle and C. Powers for sample preparation and H-L. Liu for assistance with clone construction. We thank N. Mizushima for GFP–LC3-expressing Atg5−/− and matchedwild-type MEFs, M. Komatsu and T. Ishii for p 62−/− and matched wild-type MEFs, S. Jones for ex vivo C57BL/6 MEFs, B. Lewis for mouse hepatocellular cancer lines, S. Pino for in vitro activated T cells, W. Jiang for MKLP1–GFP plasmid, K. Khanna for CEP55–eGFP plasmid, J. Lippincott-Schwartz and G. Gaietta for plasmids for FPP assay, B. Levine (UT Southwestern) for Flag-tagged BECN1-expressing plasmid, A. Khodjakov for CETN1–GFP-expressing plasmids, K. Lee for hCenexin1 antibody and the Progeria Society for cell lines. The α6F antibody to Na/K-ATPase developed by D. M. Fambrough and the H4B4 antibody to LAMP2 developed by J.T. August and J. E. K. Hildreth were obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the National Institute of Child Health and Human Development and maintained by The University of Iowa, Department of Biology. This work was supported by funding from the National Institutes of Health (GM051994 to S.D. and F32 GM084660-02 to D.B.), the W.M. Keck Foundation to S.D., the Ellison Foundation (AG-SS-1918-07) to S.D., the Department of Defense (W81XWH-08-1-0457 to S.D. and W81XWH-06-1-0140 to C-T.C.) and the Diabetes and Endocrine Resource Center (5P30DK3252025). Core resources supported by the Diabetes Endocrinology Research Center grant DK32520 were also used.
Author information
Authors and Affiliations
Contributions
C-T.C. and S.D. conceived the project and wrote the manuscript with the help of T-C.K. and D.B. The experiments on the inheritance and localization of MBds as well as some for MBd degradation were conducted by C-T.C. The experiments on MBd accumulation were conducted by C-T.C. with the help of T-C.K. and C.M.W. Investigation of the mechanisms for MBd degradation was conceived by T-C.K. and S.D., and much of the work executed by T-C.K. Autophagic flux assay, soft-agar assay of FACS-isolated cells and MBd localization in neural progenitors were conducted by D.B., who contributed substantially to the work and intellectual input on multiple aspects of this project. The reprogramming assay was conducted and analysed by T-C.K., T.T.O and S.L. The preparation of hESCs for live imaging was conducted by S.A. Tissue preparation was assisted by P.X. and J.M.H.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1454 kb)
Rights and permissions
About this article
Cite this article
Kuo, TC., Chen, CT., Baron, D. et al. Midbody accumulation through evasion of autophagy contributes to cellular reprogramming and tumorigenicity. Nat Cell Biol 13, 1214–1223 (2011). https://doi.org/10.1038/ncb2332
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2332
This article is cited by
-
An oocyte meiotic midbody cap is required for developmental competence in mice
Nature Communications (2023)
-
Secreted midbody remnants are a class of extracellular vesicles molecularly distinct from exosomes and microparticles
Communications Biology (2021)
-
Cytokinetic Abscission Regulation in Neural Stem Cells and Tissue Development
Current Stem Cell Reports (2021)
-
The Flemmingsome reveals an ESCRT-to-membrane coupling via ALIX/syntenin/syndecan-4 required for completion of cytokinesis
Nature Communications (2020)
-
Cytokinetic bridge triggers de novo lumen formation in vivo
Nature Communications (2020)