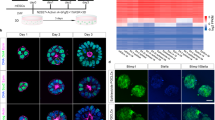
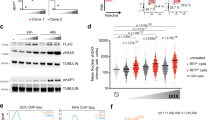
Abstract
Pluripotent stem cells exist in naive and primed states, epitomized by mouse embryonic stem cells (ESCs) and the developmentally more advanced epiblast stem cells (EpiSCs; ref. 1). In the naive state of ESCs, the genome has an unusual open conformation and possesses a minimum of repressive epigenetic marks2. In contrast, EpiSCs have activated the epigenetic machinery that supports differentiation towards the embryonic cell types3,4,5,6. The transition from naive to primed pluripotency therefore represents a pivotal event in cellular differentiation. But the signals that control this fundamental differentiation step remain unclear. We show here that paracrine and autocrine Wnt signals are essential self-renewal factors for ESCs, and are required to inhibit their differentiation into EpiSCs. Moreover, we find that Wnt proteins in combination with the cytokine LIF are sufficient to support ESC self-renewal in the absence of any undefined factors, and support the derivation of new ESC lines, including ones from non-permissive mouse strains. Our results not only demonstrate that Wnt signals regulate the naive-to-primed pluripotency transition, but also identify Wnt as an essential and limiting ESC self-renewal factor.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
07 October 2011
In the version of this article initially published online, the sequences of the primers used for Wnt2b, Wnt3, Wnt3a, Wnt4, Wnt5a and Wnt5b listed in supplementary table 5 were incorrect.
References
Nichols, J. & Smith, A. Naive and primed pluripotent states. Cell Stem Cell 4, 487–492 (2009).
Niwa, H. Open conformation chromatin and pluripotency. Genes Dev. 21, 2671–2676 (2007).
Brons, I. G. et al. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature 448, 191–195 (2007).
Tesar, P. J. et al. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature 448, 196–199 (2007).
Hayashi, K., Lopes, S. M., Tang, F. & Surani, M. A. Dynamic equilibrium and heterogeneity of mouse pluripotent stem cells with distinct functional and epigenetic states. Cell Stem Cell 3, 391–401 (2008).
Guo, G. et al. Klf4 reverts developmentally programmed restriction of ground state pluripotency. Development 136, 1063–1069 (2009).
ten Berge, D. et al. Wnt signaling mediates self-organization and axis formation in embryoid bodies. Cell Stem Cell 3, 508–518 (2008).
Sato, N., Meijer, L., Skaltsounis, L., Greengard, P. & Brivanlou, A. H. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat. Med. 10, 55–63 (2004).
Chen, B. et al. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat. Chem. Biol. 5, 100–107 (2009).
Lustig, B. et al. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol. Cell Biol. 22, 1184–1193 (2002).
Anton, R., Kestler, H. A. & Kuhl, M. β-catenin signaling contributes to stemness and regulates early differentiation in murine embryonic stem cells. FEBS Lett. 581, 5247–5254 (2007).
Kemp, C., Willems, E., Abdo, S., Lambiv, L. & Leyns, L. Expression of all Wnt genes and their secreted antagonists during mouse blastocyst and postimplantation development. Dev. Dyn. 233, 1064–1075 (2005).
Wang, Q. T. et al. A genome-wide study of gene activity reveals developmental signaling pathways in the preimplantation mouse embryo. Dev. Cell 6, 133–144 (2004).
Haegel, H. et al. Lack of β-catenin affects mouse development at gastrulation. Development 121, 3529–3537 (1995).
Ohsugi, M. et al. Expression and cell membrane localization of catenins during mouse preimplantation development. Dev. Dyn. 206, 391–402 (1996).
De Vries, W. N. et al. Maternal β-catenin and E-cadherin in mouse development. Development 131, 4435–4445 (2004).
Nichols, J., Chambers, I., Taga, T. & Smith, A. Physiological rationale for responsiveness of mouse embryonic stem cells to gp130 cytokines. Development 128, 2333–2339 (2001).
Stambolic, V., Ruel, L. & Woodgett, J. R. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr. Biol. 6, 1664–1668 (1996).
Doble, B. W. & Woodgett, J. R. GSK-3: tricks of the trade for a multi-tasking kinase. J. Cell Sci. 116, 1175–1186 (2003).
Tighe, A., Ray-Sinha, A., Staples, O. D. & Taylor, S. S. GSK-3 inhibitors induce chromosome instability. BMC Cell Biol. 8, 34 (2007).
Acevedo, N., Wang, X., Dunn, R. L. & Smith, G. D. Glycogen synthase kinase-3 regulation of chromatin segregation and cytokinesis in mouse preimplantation embryos. Mol. Reprod. Dev. 74, 178–188 (2007).
Ying, Q. L. et al. The ground state of embryonic stem cell self-renewal. Nature 453, 519–523 (2008).
Kelly, K.F. et al. β-catenin enhances Oct-4 activity and reinforces pluripotency through a TCF-independent mechanism. Cell Stem Cell 8, 214–227 (2011).
Nichols, J., Silva, J., Roode, M. & Smith, A. Suppression of Erk signalling promotes ground state pluripotency in the mouse embryo. Development 136, 3215–3222 (2009).
Gardner, R. L. & Brook, F. A. Reflections on the biology of embryonic stem (ES) cells. Int. J. Dev. Biol. 41, 235–243 (1997).
Cole, M. F., Johnstone, S. E., Newman, J. J., Kagey, M. H. & Young, R. A. Tcf3 is an integral component of the core regulatory circuitry of embryonic stem cells. Genes Dev. 22, 746–755 (2008).
Tam, W. L. et al. T-cell factor 3 regulates embryonic stem cell pluripotency and self-renewal by the transcriptional control of multiple lineage pathways. Stem Cells 26, 2019–2031 (2008).
Yi, F., Pereira, L. & Merrill, B. J. Tcf3 functions as a steady-state limiter of transcriptional programs of mouse embryonic stem cell self-renewal. Stem Cells 26, 1951–1960 (2008).
Kielman, M. F. et al. Apc modulates embryonic stem-cell differentiation by controlling the dosage of β-catenin signaling. Nat. Genet. 32, 594–605 (2002).
Hao, J., Li, T. G., Qi, X., Zhao, D. F. & Zhao, G. Q. WNT/β-catenin pathway up-regulates Stat3 and converges on LIF to prevent differentiation of mouse embryonic stem cells. Dev. Biol. 290, 81–91 (2006).
Ogawa, K., Nishinakamura, R., Iwamatsu, Y., Shimosato, D. & Niwa, H. Synergistic action of Wnt and LIF in maintaining pluripotency of mouse ES cells. Biochem. Biophys. Res. Commun. 343, 159–166 (2006).
Singla, D. K., Schneider, D. J., LeWinter, M. M. & Sobel, B. E. wnt3a but not wnt11 supports self-renewal of embryonic stem cells. Biochem. Biophys. Res. Commun. 345, 789–795 (2006).
Williams, R. L. et al. Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature 336, 684–687 (1988).
Smith, A. G. et al. Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. Nature 336, 688–690 (1988).
Hanna, J. et al. Metastable pluripotent states in NOD-mouse-derived ESCs. Cell Stem Cell 4, 513–524 (2009).
Hsieh, J. C., Rattner, A., Smallwood, P. M. & Nathans, J. Biochemical characterization of Wnt–frizzled interactions using a soluble, biologically active vertebrate Wnt protein. Proc. Natl Acad. Sci. USA 96, 3546–3551 (1999).
Willert, K. et al. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature 423, 448–452 (2003).
Mikels, A. J. & Nusse, R. Purified Wnt5a protein activates or inhibits β-catenin–TCF signaling depending on receptor context. PLoS Biol 4, e115 (2006).
Ying, Q. L., Stavridis, M., Griffiths, D., Li, M. & Smith, A. Conversion of embryonic stem cells into neuroectodermal precursors in adherent monoculture. Nat. Biotechnol. 21, 183–186 (2003).
Lamprecht, M. R., Sabatini, D. M. & Carpenter, A. E. CellProfiler: free, versatile software for automated biological image analysis. Biotechniques 42, 71–75 (2007).
Acknowledgements
These studies were supported by the Howard Hughes Medical Institute, the Erasmus MC Stem Cell Institute and grants from the California Institute of Regenerative Medicine (RC1-00133-1), the National Institutes of Health (DK67834-01) and the European Union (FP7-PEOPLE-2009-RG-256560). We thank H. Zeng for technical advice, J. Kong-A-San for assistance with blastocyst injections and R. van der Linden for assistance with FACS. We are grateful for the use of the Cellavista imager and the assistance of V. Vincent and Roche Diagnostics.
Author information
Authors and Affiliations
Contributions
D.t.B., D.K. and T.B. designed and carried out experiments, analysed data and wrote the paper. W.K., A.M., R.S. and E.E. designed and carried out experiments and analysed data. R.N. designed experiments and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1258 kb)
Rights and permissions
About this article
Cite this article
ten Berge, D., Kurek, D., Blauwkamp, T. et al. Embryonic stem cells require Wnt proteins to prevent differentiation to epiblast stem cells. Nat Cell Biol 13, 1070–1075 (2011). https://doi.org/10.1038/ncb2314
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2314
This article is cited by
-
Inhibition of DNMT3B expression in activated hepatic stellate cells overcomes chemoresistance in the tumor microenvironment of hepatocellular carcinoma
Scientific Reports (2024)
-
Glutamate secretion by embryonic stem cells as an autocrine signal to promote proliferation
Scientific Reports (2023)
-
Molecular versatility during pluripotency progression
Nature Communications (2023)
-
DNMT3B supports meso-endoderm differentiation from mouse embryonic stem cells
Nature Communications (2023)
-
CDK5RAP2 is a Wnt target gene and promotes stemness and progression of oral squamous cell carcinoma
Cell Death & Disease (2023)