Abstract
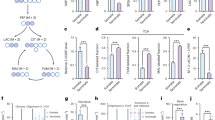
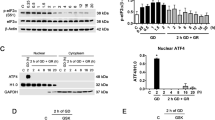
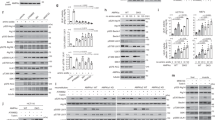
Neurons are known to have a lower glycolytic rate than astrocytes and when stressed they are unable to upregulate glycolysis1 because of low Pfkfb3 (6-phosphofructo-2-kinase/fructose-2, 6-bisphosphatase-3) activity2. This enzyme generates fructose-2,6-bisphosphate (F2,6P2)3, the most potent activator of 6-phosphofructo-1-kinase (Pfk1; ref. 4), a master regulator of glycolysis5. Here, we show that Pfkfb3 is absent from neurons in the brain cortex and that Pfkfb3 in neurons is constantly subject to proteasomal degradation by the action of the E3 ubiquitin ligase6, anaphase-promoting complex/cyclosome (APC/C)–Cdh1. By contrast, astrocytes have low APC/C–Cdh1 activity and therefore Pfkfb3 is present in these cells. Upregulation of Pfkfb3 by either inhibition of Cdh1 or overexpression of Pfkfb3 in neurons resulted in the activation of glycolysis. This, however, was accompanied by a marked decrease in the oxidation of glucose through the pentose phosphate pathway (a metabolic route involved in the regeneration of reduced glutathione7) resulting in oxidative stress and apoptotic death. Thus, by actively downregulating glycolysis by APC/C–Cdh1, neurons use glucose to maintain their antioxidant status at the expense of its utilization for bioenergetic purposes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
Almeida, A., Almeida, J., Bolaños, J. P. & Moncada, S. Different responses of astrocytes and neurons to nitric oxide: the role of glycolytically-generated ATP in astrocyte protection. Proc. Natl Acad. Sci. USA 98, 15294–15299 (2001).
Almeida, A., Moncada, S. & Bolaños, J. P. Nitric oxide switches on glycolysis through the AMP protein kinase and 6-phosphofructo-2-kinase pathway. Nature Cell Biol. 6, 45–51 (2004).
Hue, L. & Rider, M. H. Role of fructose 2, 6-bisphosphate in the control of glycolysis in mammalian tissues. Biochem. J. 245, 313–324 (1987).
Van Schaftingen, E., Lederer, B., Bartrons, R. & Hers, H. G. A kinetic study of pyrophosphate: fructose-6-phosphate phosphotransferase from potato tubers. Application to a microassay of fructose 2, 6-bisphosphate. Eur. J. Biochem. 129, 191–195 (1982).
Uyeda, K. Phosphofructokinase. Adv. Enzymol. Relat. Areas Mol. Biol. 48, 193–244 (1979).
Sudakin, V. et al. The cyclosome, a large complex containing cyclin-selective ubiquitin ligase activity, targets cyclins for destruction at the end of mitosis. Mol. Biol. Cell 6, 185–197 (1995).
Kletzien, R. F., Harris, P. K. W. & Foellmi, L. A. Glucose-6-phosphate dehydrogenase: a housekeeping enzyme subject to tissue-specific regulation by hormones, nutrients, and oxidant stress. FASEB J. 8, 174–181 (1994).
Riera, L. et al. Regulation of ubiquitous 6-phosphofructo-2-kinase by the ubiquitin-proteasome proteolytic pathway during myogenic C2C12 cell differentiation. FEBS Lett. 550, 23–29 (2003).
Pfleger, C. M. & Kirschner, M. W. The KEN box: an APC recognition signal distinct from the D box targeted by Cdh1. Genes Dev. 14, 655–65 (2000).
Visintin, R., Prinz, S. & Amon, A. CDC20 and CDH1: a family of substrate-specific activators of APC-dependent proteolysis. Science 278, 460–463 (1997).
Almeida, A., Bolanos, J. P. & Moreno, S. Cdh1/Hct1-APC is essential for the survival of postmitotic neurons. J. Neurosci. 25, 8115–8121 (2005).
Cohen, S. S. Studies on the distribution of the oxidative pathway of glucose-6-phosphate utilization. Biol. Bull. 99, 369 (1950).
Hothersall, J. S., Baquer, N. Z., Greenbaum, A. L. & McLean, P. Alternative pathways of glucose utilization in brain. Changes in the pattern of glucose utilization in brain during development and the effect of phenazine methosulphate on the integration of metabolic routes. Arch. Biochem. Biophys. 198, 478–492 (1979).
Garcia Nogales, P., Almeida, A. & Bolaños, J. P. Peroxynitrite protects neurons against nitric oxide-mediated apoptosis. A key role for glucose-6-phosphate dehydrogenase activity in neuroprotection. J. Biol. Chem. 278, 864–874 (2003).
Ben-Yoseph, O., Boxer, P. A. & Ross, B. D. Assessment of the role of the glutathione and pentose phosphate pathways in the protection of primary cerebrocortical cultures from oxidative stress. J. Neurochem. 66, 2329–2337 (1996).
Vaughn, A. E. & Deshmukh, M. Glucose metabolism inhibits apoptosis in neurons and cancer cells by redox inactivation of cytochrome c. Nature Cell Biol. 10, 1477–1483 (2008).
Tsacopoulos, M. & Magistretti, P. J. Metabolic coupling between glia and neurons. J. Neurosci. 16, 877–885 (1996).
Pellerin, L. et al. Activity-dependent regulation of energy metabolism by astrocytes: an update. Glia 55, 1251–1262 (2007).
Cidad, P., Almeida, A. & Bolaños, J. P. Inhibition of mitochondrial respiration by nitric oxide rapidly stimulates cytoprotective GLUT3-mediated glucose uptake through5′-AMP-activated protein kinase. Biochem. J. 384, 629–636 (2004).
Brummelkamp, T. R., Bernards, R. & Agami, R. A system for stable expression of short interfering RNAs in mammalian cells. Science 296, 550–553 (2002).
Reynolds, A. et al. Rational siRNA design for RNA interference. Nature Biotechnol. 22, 326–330 (2004).
Ui-Tei, K. et al. Guidelines for the selection of highly effective siRNA sequences for mammalian and chick RNA interference. Nucleic Acids Res. 32, 936–948 (2004).
Larrabee, M. G. Evaluation of the pentose phosphate pathway from 14CO2 data. Fallibility of a classic equation when applied to non-homogeneous tissues. Biochem. J. 272, 127–132 (1990).
Lowry, O. H., Rosebrough, N. J., Lewis-Farr, A. & Randall, R. J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193, 265–275 (1951).
Acknowledgements
This work was supported by Ministerio de Ciencia e Innovación (SAF2007-61492 and Consolider-Ingenio CSD2007-00020, Spain), Instituto de Salud Carlos-III (FIS06/0794 and Renevas), and Junta de Castilla y León (SA066A07 and Red de Terapia Celular y Medicina Regenerativa). We would like to thank H. Yamano for valuable help with the APC/C–Cdh1 activity assays, Carmela Gómez-Rodríguez for immunohistochemistry experiments, M. Resch for technical assistance and A. Higgs for critical evaluation of this paper.
Author information
Authors and Affiliations
Contributions
A.H.M., E.F. and C.M. performed the experiments; A.A. and J.P.B. analysed the data; A.A., S.M. and J.P.B. planned the experiments and wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 4215 kb)
Rights and permissions
About this article
Cite this article
Herrero-Mendez, A., Almeida, A., Fernández, E. et al. The bioenergetic and antioxidant status of neurons is controlled by continuous degradation of a key glycolytic enzyme by APC/C–Cdh1. Nat Cell Biol 11, 747–752 (2009). https://doi.org/10.1038/ncb1881
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1881
This article is cited by
-
Mechanosensitive channel of large conductance enhances the mechanical stretching-induced upregulation of glycolysis and oxidative metabolism in Schwann cells
Cell Communication and Signaling (2024)
-
PFKFB3 in neovascular eye disease: unraveling mechanisms and exploring therapeutic strategies
Cell & Bioscience (2024)
-
TREM2 deficiency impairs the energy metabolism of Schwann cells and exacerbates peripheral neurological deficits
Cell Death & Disease (2024)
-
Oligodendrocyte–axon metabolic coupling is mediated by extracellular K+ and maintains axonal health
Nature Neuroscience (2024)
-
AMPK role in epilepsy: a promising therapeutic target?
Journal of Neurology (2024)