Abstract
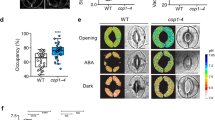
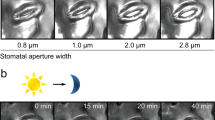
Carbon dioxide uptake and water vapour release in plants occur through stomata, which are formed by guard cells. These cells respond to light intensity, CO2 and water availability, and plant hormones1,2. The predicted increase in the atmospheric concentration of CO2 is expected to have a profound effect on our ecosystem. However, many aspects of CO2-dependent stomatal movements are still not understood3. Here we show that the ABC transporter AtABCB14 modulates stomatal closure on transition to elevated CO2. Stomatal closure induced by high CO2 levels was accelerated in plants lacking AtABCB14. Apoplastic malate has been suggested to be one of the factors mediating the stomatal response to CO2 (Refs 4,5) and indeed, exogenously applied malate induced a similar AtABCB14-dependent response as high CO2 levels. In isolated epidermal strips that contained only guard cells, malate-dependent stomatal closure was faster in plants lacking the AtABCB14 and slower in AtABCB14-overexpressing plants, than in wild-type plants, indicating that AtABCB14 catalyses the transport of malate from the apoplast into guard cells. Indeed, when AtABCB14 was heterologously expressed in Escherichia coli and HeLa cells, increases in malate transport activity were observed. We therefore suggest that AtABCB14 modulates stomatal movement by transporting malate from the apoplast into guard cells, thereby increasing their osmotic pressure.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Fan, L., Zhao, Z. & Assmann, S. M. Guard cells: a dynamic signaling model. Curr. Opin. Plant Biol. 7, 537–546 (2004).
Hetherington, A. M. Guard cell signaling. Cell 107, 711–714 (2001).
Schroeder, J. I. et al. Guard cell ABA and CO2 signaling network updates and Ca2+ sensor priming hypothesis. Curr. Opin. Plant Biol. 9, 654–663 (2006).
Hedrich, R. & Marten, I. Malate-induced feedback regulation of plasma membrane anion channels could provide a CO2 sensor to guard cells. EMBO J. 12, 897–901 (1993).
Hedrich, R. et al. Malate-sensitive anion channels enable guard cells to sense changes in the ambient CO2 concentration. Plant J. 6, 741–748 (1994).
Holland, I. B., Cole, S. P. C., Kuchler, K. & Higgins, C. F. ABC Proteins: From Bacteria to Man (Academic Press, Amsterdam, 2003).
Martinoia, E. et al. Multifunctionality of plant ABC transporters – more than just detoxifiers. Planta 214, 345–355 (2002).
Rea, P. A. Plant ATP -binding cassette transporters. Annu. Rev. Plant Biol. 58, 347–375 (2007).
Verrier, P. J. et al. Plant ABC proteins - a unified nomenclature and updated inventory. Trends Plant Sci. 13, 151–159 (2008).
Gaedeke, N. et al. The Arabidopsis thaliana ABC transporter AtMRP5 controls root development and stomata movement. EMBO J. 20, 1875–1887 (2001).
Klein, M. et al. The multidrug resistance ABC transporter AtMRP5 is involved in guard cell hormonal signaling and drought tolerance. Plant J. 33, 119–129 (2003).
Suh, S. et al. The ATP-binding cassette transporter AtMRP5 modulates anion and Ca2+ channel activities in Arabidopsis guard cells. J. Biol. Chem. 282, 1916–1924 (2007).
Dewitt, N. D, Hong, B., Sussman, M. R. & Harper, J. F. Targeting of two Arabidopsis H+-ATPase isoforms to the plasma membrane. Plant Physiol. 112, 833–844 (1996).
Kobae, Y. et al. Loss of AtPDR8, a plasma membrane ABC transporter of Arabidopsis thaliana causes hypersensitive cell death upon pathogen infection. Plant Cell Physiol. 47, 309–318 (2006).
Schroeder, J. I., Allen, G. J., Hugouvieux, V., Kwak, J. M. & Waner, D. Guard cell signal transduction. Annu. Rev. Plant Physiol. Plant Mol. Biol. 52, 627–658 (2001).
Schroeder, J. I., Kwak, J. M. & Allen, G. J., Guard cell abscisic acid signal transduction network and engineering of plant drought hardiness. Nature 410, 327–330 (2001).
MacRobbie, E. A. C. Signal transduction and ion channels in guard cells. Phil. Trans. Roy. Soc. London. 1374, 1475–1488 (1998).
Vavasseur, A. & Raghavendra, A. S. Guard cell metabolism and CO2 sensing. New Phytol. 165, 665–682 (2005).
Young, J. J. et al. CO2 signaling in guard cells: calcium sensitivity response modulation, a Ca2+-independent phase, and CO2 insensitivity of the gca2 mutant. Proc. Natl Acad. Sci. 103, 7506–7511 (2006).
Hashimoto, M. et al. Arabidopsis HT1 kinase controls stomatal movements in response to CO2 . Nature Cell Biol. 8, 391–397 (2006).
Hedrich, R. et al. Changes in apoplastic pH and membrane potential in leaves in relation to stomatal responses to CO2, malate, abscisic acid or interruption of water supply. Planta 213, 594–601 (2001).
Raschke, K. Alternation of the slow with the quick anion conductance in whole guard cells effected by external malate. Planta 217, 651–657 (2003).
Schroeder, J. I. Anion channels as central mechanisms for signal transduction in guard cells and putative functions in roots for plant-soil interactions. Plant Mol. Biol. 28, 353–361 (1995).
Keller, B. U., Hedrich, R., & Raschke, K. Voltage-dependent anion channels in the plasma membrane of guard cells. Nature 341, 450–453 (1989).
Negi, J. et al. CO2 regulator SLAC1 and its homologues are essential for anion homeostasis in plant cells. Nature 452, 483–486 (2008).
Vahisalu, T. et al. SLAC1 is required for plant guard cell S-type anion channel function in stomatal signaling. Nature 452, 487–491 (2008).
Geisler, M. et al. Cellular efflux of auxin catalyzed by the Arabidopsis MDR/PGP transporter AtPGP1. Plant J. 44, 179–194 (2005).
Hunke, S., Döse, S., & Schneider, E. Vanadate and bafilomycin A1 are potent inhibitors of the ATPase activity of the reconstituted bacterial ATP-binding cassette transporter for maltose (MalFGK2). Biochem. Biophys. Res. Commun. 216, 589–594 (1995).
Hurth, M. A. et al. Impaired pH homeostasis in Arabidopsis lacking the vacuolar dicarboxylate transporter and analysis of carboxylic acid transport across the tonoplast. Plant Physiol. 137, 901–910 (2005).
Van Kirk, C. A. & Raschke, K. Release of malate from epidermal strips during stomatal closure. Plant Physiol. 61, 474–475 (1978).
Abel, S. & Theologis, A. Transient gene expression in protoplast of Arabidopsis thaliana. Methods Mol. Biol. 82, 209–217 (1998).
Hwang, J. U. et al. Actin filaments modulate both stomatal opening and inward K+-channel activities in guard cells of Vicia faba L. Plant Physiol. 115, 335–342 (1997).
Endler, A. et al. Identification of a vacuolar sucrose transporter in Hordeum vulgare and Arabidopsis thaliana by a tonoplast proteomic approach. Plant Physiol. 141, 196–207 (2006).
Jeong, J. et al. A nodule-specific dicarboxylate transporter from alder is a member of the peptide transporter family. Plant Physiol. 134, 969–978 (2004).
Kimoto, E. et al. Efflux transport of N-monodesethylamiodarone by the human intestinal cell-line Caco-2 cells. Drug Metab. Pharmacokinet. 22, 307–312 (2007).
Acknowledgements
We thank N. Amrhein, Swiss Federal School of Technology for reading the manuscript, A. Vavasseur for helpful discussion on gas-exchange measurements and M. Schellenberg for making section images of AtABCB14:GUS. This project was supported by the Global Research Laboratory programme of the Ministry of Science and Technology of Korea. The work in the laboratory of E.M. was partially supported by the Swiss National Foundation.
Author information
Authors and Affiliations
Contributions
M.L. performed most of the experiments; Y.C. and J.-Y.Y. assisted with the HeLa cell experiments; B.B. contributed to the gas-exchange experiment; Y.-Y.K. and M.M. contributed to the membrane fractionation experiment; B.J. contributed to the localization study; E.M. and Y.L. designed the experiments and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures S1, S2, S3, S4, S5 and S6 (PDF 978 kb)
Rights and permissions
About this article
Cite this article
Lee, M., Choi, Y., Burla, B. et al. The ABC transporter AtABCB14 is a malate importer and modulates stomatal response to CO2. Nat Cell Biol 10, 1217–1223 (2008). https://doi.org/10.1038/ncb1782
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1782
This article is cited by
-
Overexpression of chloroplastic Zea mays NADP-malic enzyme (ZmNADP-ME) confers tolerance to salt stress in Arabidopsis thaliana
Photosynthesis Research (2023)
-
Arabidopsis guard cell chloroplasts import cytosolic ATP for starch turnover and stomatal opening
Nature Communications (2022)
-
Belgian endive-derived biostimulants promote shoot and root growth in vitro
Scientific Reports (2022)
-
Molecular response and evolution of plant anion transport systems to abiotic stress
Plant Molecular Biology (2022)
-
Elevated [CO2] mitigates the impacts of heat stress in eucalyptus seedlings
Theoretical and Experimental Plant Physiology (2022)