Abstract
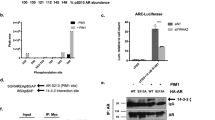
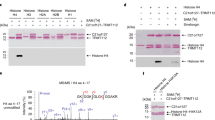
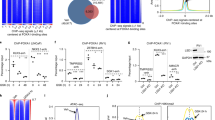
Posttranslational modifications of histones such as methylation, acetylation and phosphorylation regulate chromatin structure and gene expression. Here we show that protein-kinase-C-related kinase 1 (PRK1) phosphorylates histone H3 at threonine 11 (H3T11) upon ligand-dependent recruitment to androgen receptor target genes. PRK1 is pivotal to androgen receptor function because PRK1 knockdown or inhibition impedes androgen receptor-dependent transcription. Blocking PRK1 function abrogates androgen-induced H3T11 phosphorylation and inhibits androgen-induced demethylation of histone H3. Moreover, serine-5-phosphorylated RNA polymerase II is no longer observed at androgen receptor target promoters. Phosphorylation of H3T11 by PRK1 accelerates demethylation by the Jumonji C (JmjC)-domain-containing protein JMJD2C. Thus, phosphorylation of H3T11 by PRK1 establishes a novel chromatin mark for gene activation, identifying PRK1 as a gatekeeper of androgen receptor-dependent transcription. Importantly, levels of PRK1 and phosphorylated H3T11 correlate with Gleason scores of prostate carcinomas. Finally, inhibition of PRK1 blocks proliferation of androgen receptor-induced tumour cell proliferation, making PRK1 a promising therapeutic target.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Strahl, B. D. & Allis, C. D. The language of covalent histone modifications. Nature 403, 41–45 (2000).
Phatnani, H. P. & Greenleaf, A. L. Phosphorylation and functions of the RNA polymerase II CTD. Genes Dev. 20, 2922–2936 (2006).
Metzger, E. et al. LSD1 demethylates repressive histone marks to promote androgen receptor-dependent transcription. Nature 437, 436–439 (2005).
Wissmann, M. et al. Cooperative demethylation by JMJD2C and LSD1 promotes androgen receptor-dependent gene expression. Nature Cell Biol. 9, 347–353 (2007).
Kang, Z., Pirskanen, A., Janne, O. A. & Palvimo, J. J. Involvement of proteasome in the dynamic assembly of the androgen receptor transcription complex. J. Biol. Chem. 277, 48366–48371 (2002).
Metzger, E. et al. A novel inducible transactivation domain in the androgen receptor: implications for PRK in prostate cancer. EMBO J. 22, 270–280 (2003).
Preuss U., Landsberg G. & Scheidtmann K. H. Novel mitosis-specific phosphorylation of histone H3 at Thr11 mediated by Dlk/ZIP kinase. Nucleic Acids Res. 31, 878–885 (2003).
Tachibana M., Sugimoto K., Fukushima T. & Shinkai Y. SET domain-containing protein, G9a, is a novel lysine-preferring mammalian histone methyltransferase with hyperactivity and specific selectivity to lysines 9 and 27 of histone H3. J. Biol. Chem. 276, 25309–25317 (2001).
Ogawa H., Ishiguro K., Gaubatz S., Livingston D. M. & Nakatani Y. A complex with chromatin modifiers that occupies E2F- and Myc-responsive genes in G0 cells. Science 296, 1132–1136 (2002).
Baek S. H. et al. Ligand-specific allosteric regulation of coactivator functions of androgen receptor in prostate cancer cells. Proc. Natl Acad. Sci. USA 103, 3100–3105 (2006).
Kononen J. et al. Tissue microarrays for high-throughput molecular profiling of tumour specimens. Nature Med. 4, 844–847 (1998).
Kahl, P. et al. Androgen receptor coactivators lysine-specific histone demethylase 1 and four and a half LIM domain protein 2 predict risk of prostate cancer recurrence. Cancer Res. 66, 11341–11347 (2006).
Ng S. S. et al. Crystal structures of histone demethylase JMJD2A reveal basis for substrate specificity. Nature 448, 87–91 (2007).
Zippo A., De Robertis A., Serafini R. & Oliviero S. PIM1-dependent phosphorylation of histone H3 at serine 10 is required for MYC-dependent transcriptional activation and oncogenic transformation. Nature Cell Biol. 9, 932–944 (2007).
Garcia-Bassets I. et al. Histone methylation-dependent mechanisms impose ligand dependency for gene activation by nuclear receptors. Cell 128, 505–518 (2007).
Dai, J., Sultan, S., Taylor, S. S. & Higgins, J. M. The kinase haspin is required for mitotic histone H3 Thr 3 phosphorylation and normal metaphase chromosome alignment. Genes Dev. 19, 472–488 (2005).
Shang, Y., Myers, M. & Brown, M. Formation of the androgen receptor transcription complex. Mol. Cell 9, 601–610 (2002).
Wiznerowicz, M. & Trono, D. Conditional suppression of cellular genes: lentivirus vector-mediated drug-inducible RNA interference. J. Virol. 77, 8957–8961 (2003).
Dong, L. Q. et al. Phosphorylation of protein kinase N by phosphoinositide-dependent protein kinase-1 mediates insulin signals to the actin cytoskeleton. Proc. Natl Acad. Sci. USA 97, 5089–5094 (2000).
O'Neill, T. E., Roberge, M. & Bradbury, E. M. Nucleosome arrays inhibit both initiation and elongation of transcripts by bacteriophage T7 RNA polymerase. J. Mol. Biol. 223, 67–78 (1992).
Schroder, F. H. et al. The TNM classification of prostate cancer. Prostate Suppl. 4, 129–138 (1992).
Hsing, A. W., Tsao L. & Devesa S. S. International trends and patterns of prostate cancer incidence and mortality. Int. J. Cancer 85, 60–67 (2000).
Acknowledgements
We thank C. Beisenherz-Huss, C. Schächtele (ProQinase GmbH, Freiburg) and R. Schneider for providing reagents. We are obliged to T. Günther, H. Greschik, J. M. Müller and S. Naumovitz for discussions. We thank F. Klott for technical assistance. This work was supported by grants from the National Institutes of Health to J.M.G.H., the Deutsche Forschungsgemeinschaft, the Dr Hans Messner Stiftung and Deutsche Krebshilfe to R.S.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Rights and permissions
About this article
Cite this article
Metzger, E., Yin, N., Wissmann, M. et al. Phosphorylation of histone H3 at threonine 11 establishes a novel chromatin mark for transcriptional regulation. Nat Cell Biol 10, 53–60 (2008). https://doi.org/10.1038/ncb1668
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1668
This article is cited by
-
Chromatin balances cell redox and energy homeostasis
Epigenetics & Chromatin (2023)
-
Targeting a splicing-mediated drug resistance mechanism in prostate cancer by inhibiting transcriptional regulation by PKCβ1
Oncogene (2022)
-
Metabolic phosphatase moonlights for proteins
Nature Cell Biology (2022)
-
RB1 loss in castration-resistant prostate cancer confers vulnerability to LSD1 inhibition
Oncogene (2022)
-
Dynamic changes of histone methylation in mammalian oocytes and early embryos
Histochemistry and Cell Biology (2022)