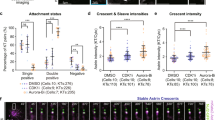
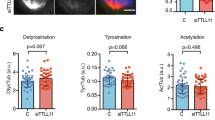
Abstract
Defects in chromosome–microtubule attachment trigger spindle-checkpoint activation and delay mitotic progression1,2. How microtubule attachment is sensed and integrated into the steps of checkpoint-signal amplification is poorly understood. In a functional genomic screen targeting human kinases and phosphatases, we identified a microtubule affinity-regulating kinase kinase, TAO1 (also known as MARKK) as an important regulator of mitotic progression, required for both chromosome congression and checkpoint-induced anaphase delay. TAO1 interacts with the checkpoint kinase BubR1 and promotes enrichment of the checkpoint protein Mad2 at sites of defective attachment, providing evidence for a regulatory step that precedes the proposed Mad2–Mad1 dependent checkpoint-signal amplification step3. We propose that the dual functions of TAO1 in regulating microtubule dynamics and checkpoint signalling may help to coordinate the establishment and monitoring of correct congression of chromosomes, thereby protecting genomic stability in human cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Bharadwaj, R. & Yu, H. The spindle checkpoint, aneuploidy, and cancer. Oncogene 23, 2016–2027 (2004).
Taylor, S. S., Scott, M. I. & Holland, A. J. The spindle checkpoint: a quality control mechanism which ensures accurate chromosome segregation. Chromosome Res. 12, 599–616 (2004).
Yu, H. Structural activation of Mad2 in the mitotic spindle checkpoint: the two-state Mad2 model versus the Mad2 template model. J. Cell Biol. 173, 153–157 (2006).
Chan, G. K., Liu, S. T. & Yen, T. J. Kinetochore structure and function. Trends Cell Biol. 15, 589–598 (2005).
Basto, R., Gomes, R. & Karess, R. E. Rough deal and Zw10 are required for the metaphase checkpoint in Drosophila. Nature Cell Biol. 2, 939–943 (2000).
Habu, T., Kim, S. H., Weinstein, J. & Matsumoto, T. Identification of a MAD2-binding protein, CMT2, and its role in mitosis. EMBO J. 21, 6419–6428 (2002).
Lou, Y. et al. NEK2A interacts with MAD1 and possibly functions as a novel integrator of the spindle checkpoint signaling. J. Biol. Chem. 279, 20049–20057 (2004).
Hutchison, M., Berman, K. S. & Cobb, M. H. Isolation of TAO1, a protein kinase that activates MEKs in stress-activated protein kinase cascades. J. Biol. Chem. 273, 28625–28632 (1998).
Takenaka, K., Moriguchi, T. & Nishida, E. Activation of the protein kinase p38 in the spindle assembly checkpoint and mitotic arrest. Science 280, 599–602 (1998).
Mikhailov, A., Shinohara, M. & Rieder, C. L. Topoisomerase II and histone deacetylase inhibitors delay the G2/M transition by triggering the p38 MAPK checkpoint pathway. J. Cell Biol. 166, 517–526 (2004).
Matsusaka, T. & Pines, J. Chfr acts with the p38 stress kinases to block entry to mitosis in mammalian cells. J. Cell Biol. 166, 507–516 (2004).
Yustein, J. T. et al. Comparative studies of a new subfamily of human Ste20-like kinases: homodimerization, subcellular localization, and selective activation of MKK3 and p38. Oncogene 22, 6129–6141 (2003).
Pines, J. Mitosis: a matter of getting rid of the right protein at the right time. Trends Cell Biol. 16, 55–63 (2006).
Pinsky, B. A. & Biggins, S. The spindle checkpoint: tension versus attachment. Trends Cell Biol. 15, 486–493 (2005).
Timm, T. et al. MARKK, a Ste20-like kinase, activates the polarity-inducing kinase MARK/PAR-1. EMBO J. 22, 5090–5101 (2003).
Taylor, S. S. & McKeon, F. Kinetochore localization of murine Bub1 is required for normal mitotic timing and checkpoint response to spindle damage. Cell 89, 727–735 (1997).
Gorbsky, G. J., Chen, R. H. & Murray, A. W. Microinjection of antibody to Mad2 protein into mammalian cells in mitosis induces premature anaphase. J. Cell Biol. 141, 1193–1205 (1998).
Johnson, V. L., Scott, M. I., Holt, S. V., Hussein, D. & Taylor, S. S. Bub1 is required for kinetochore localization of BubR1, Cenp-E, Cenp-F and Mad2, and chromosome congression. J. Cell Sci. 117, 1577–1589 (2004).
Meraldi, P. & Sorger, P. K. A dual role for Bub1 in the spindle checkpoint and chromosome congression. EMBO J. 24, 1621–1633 (2005).
Morrow, C. J. et al. Bub1 and aurora B cooperate to maintain BubR1-mediated inhibition of APC/CCdc20. J. Cell Sci. 118, 3639–3652 (2005).
Lampson, M. A. & Kapoor, T. M. The human mitotic checkpoint protein BubR1 regulates chromosome-spindle attachments. Nature Cell Biol. 7, 93–98 (2005).
Harrington, E. A. et al. VX-680, a potent and selective small-molecule inhibitor of the Aurora kinases, suppresses tumor growth in vivo. Nature Med. 10, 262–267 (2004).
Zeitlin, S. G., Shelby, R. D. & Sullivan, K. F. CENP-A is phosphorylated by Aurora B kinase and plays an unexpected role in completion of cytokinesis. J. Cell Biol. 155, 1147–1157 (2001).
Meraldi, P., Draviam, V. M. & Sorger, P. K. Timing and checkpoints in the regulation of mitotic progression. Dev. Cell 7, 45–60 (2004).
Vigneron, S. et al. Kinetochore localization of spindle checkpoint proteins: who controls whom? Mol. Biol. Cell 15, 4584–4596 (2004).
Wassmann, K., Liberal, V. & Benezra, R. Mad2 phosphorylation regulates its association with Mad1 and the APC/C. EMBO J. 22, 797–806 (2003).
Ditchfield, C. et al. Aurora B couples chromosome alignment with anaphase by targeting BubR1, Mad2, and Cenp-E to kinetochores. J. Cell Biol. 161, 267–280 (2003).
Zhou, B. B. & Elledge, S. J. The DNA damage response: putting checkpoints in perspective. Nature 408, 433–439 (2000).
Wollman, R. et al. Efficient chromosome capture requires a bias in the 'search-and-capture' process during mitotic-spindle assembly. Curr Biol. 15, 828–832 (2005).
Silva, J. M. et al. Second-generation shRNA libraries covering the mouse and human genomes. Nature Genet. 37, 1281–1288 (2005).
Draviam, V. M., Shapiro, I., Aldridge, B. & Sorger, P. K. Misorientation and reduced stretching of aligned sister kinetochores promote chromosome missegregation in EB1- or APC-depleted cells. EMBO J. 25, 2814–2827 (2006).
Acknowledgements
We thank H. Yu, S. Taylor, W. Earnshaw, H. Kung, N. Gray, D.Allis and J. Jin for gifts of reagents, M. Vidal for providing access to their BioRobot platform, S. Lyman and R. King for communicating unpublished results and assistance with the development of the taxol screening assay, C. Shamu for access to the ICCB-Longwood screening facilities, and members of the Elledge lab for their critical reading of the manuscript. F.S. is a fellow of the Helen Hay Whitney Foundation. M.S. is supported by a post-doctoral fellowship from the American Cancer Society. The siRNA and ICCB-Longwood resources used were funded in part by a National Cancer Institute grant CA78048 (Tim Mitchison). This work was supported by grants from the National Institutes of Health (NIH) and Department of Defense to S.J.E., by grants from the NIH to J.W.H and to P.K.S. S.J.E. and G.J.H. are investigators with the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures S1, S2, S3, S4 and S5 (PDF 329 kb)
Supplementary Information
Supplementary Table S1 (PDF 37583 kb)
Supplementary Information
Supplementary Table S2 (PDF 447 kb)
Supplementary Information
Supplementary Table S3 (PDF 693 kb)
Supplementary Information
Supplementary Table S4 (PDF 288 kb)
Rights and permissions
About this article
Cite this article
Draviam, V., Stegmeier, F., Nalepa, G. et al. A functional genomic screen identifies a role for TAO1 kinase in spindle-checkpoint signalling. Nat Cell Biol 9, 556–564 (2007). https://doi.org/10.1038/ncb1569
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1569
This article is cited by
-
TAOK1 negatively regulates IL-17-mediated signaling and inflammation
Cellular & Molecular Immunology (2018)
-
A novel de novo microdeletion at 17q11.2 adjacent to NF1 gene associated with developmental delay, short stature, microcephaly and dysmorphic features
Molecular Cytogenetics (2016)
-
RNAi-based functional selection identifies novel cell migration determinants dependent on PI3K and AKT pathways
Nature Communications (2014)
-
Interlaboratory reproducibility of large-scale human protein-complex analysis by standardized AP-MS
Nature Methods (2013)
-
A genome-wide homologous recombination screen identifies the RNA-binding protein RBMX as a component of the DNA-damage response
Nature Cell Biology (2012)