Abstract
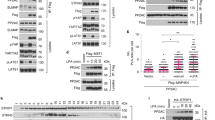
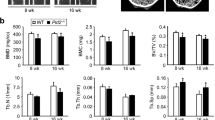
Growth hormone binds to its membrane receptor (GHR), whereby it regulates many cellular functions, including proliferation, differentiation and chemotaxis. However, although the activation of growth hormone-mediated signalling is well understood, the precise mechanism responsible for its regulation has not been elucidated. Here, we demonstrate that phospholipase Cγ1 (PLCγ1) modulates the action of growth hormone-mediated signalling by interacting with tyrosine kinase Jak2 (janus kinase 2) in a growth hormone-dependent manner. In the absence of PLCγ1 (PLCγ1−/−), growth hormone-induced JAK2 and STAT5 phosphorylation significantly increased in mouse embryonic fibroblasts (MEFs). Furthermore, the re-expression of PLCγ1 reduced growth hormone-induced Jak2 activation. Growth hormone-induced Jak2 phosphorylation was enhanced by siRNA-specific knockdown of PLCγ1. Interestingly, PLCγ1 physically linked Jak2 and protein tyrosine phosphatase-1B (PTP-1B) by binding to both using different domains, and this process was implicated in the modulation of cytokine signalling through Jak2. In addition, in PLCγ1−/− MEFs, growth hormone-dependent c-Fos activation was upregulated and growth hormone-induced proliferation was potentiated. These results suggest that PLCγ1 has a key function in the regulation of growth hormone-mediated signalling by negatively regulating Jak2 activation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Lupu, F., Terwilliger, J. D., Lee, K., Segre, G. V. & Efstratiadis, A. Roles of growth hormone and insulin-like growth factor 1 in mouse postnatal growth. Dev. Biol. 229, 141–162 (2001).
Argetsinger, L. S. et al. Identification of JAK2 as a growth hormone receptor-associated tyrosine kinase. Cell 74, 237–244 (1993).
Herrington, J., & Carter-Su, C. Signaling pathways activated by the growth hormone receptor. Trends Endocrinol. Metab. 12, 252–257 (2001).
Herrington, J., Smit, L. S., Schwartz, J. & Carter-Su, C. The role of STAT proteins in growth hormone signaling. Oncogene 19, 2585–2597 (2000).
Fain, J. N. et al. Regulation of phosphoinositide-specific phospholipase C. Biochim. Biophys. Acta. 1053, 81–88 (1990).
Rhee, S. G. & Bae, Y. S. Regulation of phosphoinositide-specific phospholipase C isozymes. J. Biol. Chem. 272, 15045–15048 (1997).
Patterson, R. L. et al. Phospholipase C-γ is required for agonist-induced Ca2+ entry. Cell 111, 529–541 (2002).
Ye, K. et al. Phospholipase C γ 1 is a physiological guanine nucleotide exchange factor for the nuclear GTPase PIKE. Nature 415, 541–544 (2002).
Choi, J. H. et al. Phospholipase C-γ1 is a guanine nucleotide exchange factor for dynamin-1 and enhances dynamin-1-dependent epidermal growth factor receptor endocytosis. J. Cell Sci. 117, 3785–3795 (2004).
Rogers, S. A. & Hammerman, M. R. Growth hormone activates phospholipase C in proximal tubular basolateral membranes from canine kidney. Proc. Natl Acad. Sci. USA 86, 6363–6366 (1989).
Doglio, A., Dani, C., Grimaldi, P. & Ailhaud, G. Growth hormone stimulates c-fos gene expression by means of protein kinase C without increasing inositol lipid turnover. Proc. Natl Acad. Sci. USA. 86, 1148–1152 (1989).
Argetsinger, L. S. & Carter-Su, C. Mechanism of signaling by growth hormone receptor. Physiol. Rev. 76, 1089–1107 (1996).
Sjoholm, A. et al. Rapid Ca2+ influx and diacylglycerol synthesis in growth hormone-mediated islet β-cell mitogenesis. J. Biol. Chem. 275, 21033–21040 (2000).
Irvin, B. J., Williams, B. L., Nilson, A. E., Maynor, H. O. & Abraham, R. T. Pleiotropic contributions of phospholipase C-γ1 (PLC-γ1) to T-cell antigen receptor-mediated signaling: reconstitution studies of a PLC-γ1-deficient Jurkat T-cell line. Mol. Cell. Biol. 20, 9149–9161 (2000).
Ji, Q. S. et al. Essential role of the tyrosine kinase substrate phospholipase C-γ1 in mammalian growth and development. Proc. Natl Acad. Sci. USA 94, 2999–3003 (1997).
Strous, G. J., van, Kerkhof. P., Govers, R., Ciechanover, A. & Schwartz, A. L. The ubiquitin conjugation system is required for ligand-induced endocytosis and degradation of the growth hormone receptor. EMBO J. 15, 3806–3812 (1996).
Narazaki, M. et al. Activation of JAK2 kinase mediated by the interleukin 6 signal transducer gp130. Proc. Nat. Acad. Sci. USA 91, 2285–2289 (1994).
Myers, M. P. et al. TYK2 and JAK2 are substrates of protein-tyrosine phosphatase 1B. J. Biol. Chem. 276, 47771–47774 (2001).
Gu, F. et al. Protein tyrosine phosphatase 1B attenuates growth hormone-mediated JAK2-STAT signaling Mol. Cell. Biol. 23, 3753–3762 (2003).
Watanabe, S., Itoh, T. & Arai, K. JAK2 is essential for activation of c-fos and c-myc promoters and cell proliferation through the human granulocyte-macrophage colony-stimulating factor receptor in BA/F3 cells. J. Biol. Chem. 271, 12681–12686 (1996).
Liu, J. P., Baker, J., Perkins, A. S., Robertson, E. J. & Efstratiadis, A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell 75, 59–72 (1993).
Heinrich, P. C. et al. Principles of IL-6-type cytokine signaling and its regulation. Biochem. J. 374, 1–20 (2003).
Elchebly, M. et al. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science 283, 1544–1548 (1999).
Klaman, L. D. et al. Increased energy expenditure, decreased adiposity, and tissue-specific insulin sensitivity in protein-tyrosine phosphatase 1B-deficient mice. Mol. Cell. Biol. 20, 5479–5489 (2000).
Kofoed, E. M. et al. Growth hormone insensitivity associated with a STAT5b mutation. N. Engl. J. Med. 349, 1139–1147 (2003).
Cressman, D. E. et al. Liver failure and defective hepatocyte regeneration in interleukin-6-defcient mice. Science 274, 1379–1383 (1996).
Waters, M. J., Hoang, H. N., Fairlie, D. P., Pelekanos, R. A. & Brown, R. J. New insights into growth hormone action. J. Mol. Endocrinol. 36, 1–7 (2006).
Sangwan, Y. et al. Protein-tyrosine phosphatase 1B deficiency protects against Fas-induced hepatic failure. J. Biol. Chem. 281, 221–228 (2006).
Liu, F. & Chernoff, J. Protein tyrosine phosphatase 1B interacts with and is tyrosine phosphorylated by the epidermal growth factor receptor. Biochem J. 327, 139–145 (1997).
Peng, B. et al. CPAP is a novel stat5-interacting cofactor that augments stat5-mediated transcriptional activity. Mol. Endocrinol. 16, 2019–2033 (2002).
Acknowledgements
This study was supported by the National R&D Program for Fusion Strategy of Advanced Technologies of Ministry of Commerce, Industry and Energy (MOCIE). This work was supported by the National Research Laboratory of the Korea Science and Engineering Foundation (grant M10600000281-06J0000-28110) and Brain Korea21 Program of the Ministry of Education of Korea.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary figures S1, S2, S3 and Supplementary methods (PDF 400 kb)
Rights and permissions
About this article
Cite this article
Choi, J., Kim, H., Kim, SH. et al. Phospholipase Cγ1 negatively regulates growth hormone signalling by forming a ternary complex with Jak2 and protein tyrosine phosphatase-1B. Nat Cell Biol 8, 1389–1397 (2006). https://doi.org/10.1038/ncb1509
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1509
This article is cited by
-
Epo-induced erythroid maturation is dependent on Plcγ1 signaling
Cell Death & Differentiation (2015)
-
Ptp1b
AfCS-Nature Molecule Pages (2008)
-
Recent advances in growth hormone signaling
Reviews in Endocrine and Metabolic Disorders (2006)