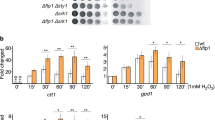
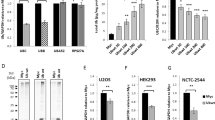
Abstract
FOXO (Forkhead box O) transcription factors are important regulators of cellular metabolism, cell-cycle progression and cell death. FOXO activity is regulated by multiple post-translational modifications, including phosphorylation, acetylation and polyubiquitination. Here, we show that FOXO becomes monoubiquitinated in response to increased cellular oxidative stress, resulting in its re-localization to the nucleus and an increase in its transcriptional activity. Deubiquitination of FOXO requires the deubiquitinating enzyme USP7/HAUSP (herpesvirus-associated ubiquitin-specific protease), which interacts with and deubiquitinates FOXO in response to oxidative stress. Oxidative stress-induced ubiquitination and deubiquitination by USP7 do not influence FOXO protein half-life. However, USP7 does negatively regulate FOXO transcriptional activity towards endogenous promoters. Our results demonstrate a novel mechanism of FOXO regulation and indicate that USP7 has an important role in regulating FOXO-mediated stress responses.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Katoh, M. Human FOX gene family (Review). Int. J. Oncol. 25, 1495–1500 (2004).
Tran, H. et al. DNA repair pathway stimulated by the forkhead transcription factor FOXO3a through the Gadd45 protein. Science 296, 530–534 (2002).
Kops, G. J. et al. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature 419, 316–321 (2002).
Burgering, B. M. & Kops, G. J. Cell cycle and death control: long live Forkheads. Trends Biochem. Sci. 27, 352–360 (2002).
Brunet, A. et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96, 857–868 (1999).
Kops, G. J. et al. Direct control of the Forkhead transcription factor AFX by protein kinase B. Nature 398, 630–634 (1999).
Tran, H., Brunet, A., Griffith, E. C. & Greenberg, M. E. The many forks in FOXO's road. Sci STKE 2003, RE5 (2003).
De Ruiter, N. D., Burgering, B. M. & Bos, J. L. Regulation of the Forkhead transcription factor AFX by Ral-dependent phosphorylation of threonines 447 and 451. Mol. Cell. Biol. 21, 8225–8235 (2001).
Essers, M. A. et al. FOXO transcription factor activation by oxidative stress mediated by the small GTPase Ral and JNK. EMBO J. 23, 4802–4812 (2004).
Wang, M. C., Bohmann, D. & Jasper, H. JNK extends life span and limits growth by antagonizing cellular and organism-wide responses to insulin signaling. Cell 121, 115–125 (2005).
Oh, S. W. et al. JNK regulates lifespan in Caenorhabditis elegans by modulating nuclear translocation of forkhead transcription factor/DAF-16. Proc. Natl Acad. Sci. USA 102, 4494–4499 (2005).
Nanji, M., Hopper, N. A. & Gems, D. LET-60 RAS modulates effects of insulin/IGF-1 signaling on development and aging in Caenorhabditis elegans. Aging Cell 4, 235–245 (2005).
Mahmud, D. L. et al. Phosphorylation of forkhead transcription factors by erythropoietin and stem cell factor prevents acetylation and their interaction with coactivator p300 in erythroid progenitor cells. Oncogene 21, 1556–1562 (2002).
Fukuoka, M. et al. Negative regulation of forkhead transcription factor AFX (Foxo4) by CBP-induced acetylation. Int. J. Mol. Med. 12, 503–508 (2003).
van der Horst, A. et al. FOXO4 is acetylated upon peroxide stress and deacetylated by the longevity protein hSir2(SIRT1). J. Biol. Chem. 279, 28873–28879 (2004).
Brunet, A. et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 303, 2011–2015 (2004).
Motta, M. C. et al. Mammalian SIRT1 represses forkhead transcription factors. Cell 116, 551–563 (2004).
Daitoku, H. et al. Silent information regulator 2 potentiates Foxo1-mediated transcription through its deacetylase activity. Proc. Natl Acad. Sci. USA 101, 10042–10047 (2004).
Huang, H. et al. Skp2 inhibits FOXO1 in tumor suppression through ubiquitin-mediated degradation. Proc. Natl Acad. Sci. USA 102, 1649–1654 (2005).
Matsuzaki, H., Daitoku, H., Hatta, M., Tanaka, K. & Fukamizu, A. Insulin-induced phosphorylation of FKHR (Foxo1) targets to proteasomal degradation. Proc. Natl Acad. Sci. USA 100, 11285–11290 (2003).
Plas, D. R. & Thompson, C. B. Akt activation promotes degradation of tuberin and FOXO3a via the proteasome. J. Biol. Chem. 278, 12361–12366 (2003).
Aoki, M., Jiang, H. & Vogt, P. K. Proteasomal degradation of the FoxO1 transcriptional regulator in cells transformed by the P3k and Akt oncoproteins. Proc. Natl Acad. Sci. USA 101, 13613–13617 (2004).
Onishi, Y. et al. Identification of mono-ubiquitinated LDH-A in skeletal muscle cells exposed to oxidative stress. Biochem. Biophys. Res. Commun. 336, 799–806 (2005).
Cao, C. et al. Ubiquitination and degradation of the Arg tyrosine kinase is regulated by oxidative stress. Oncogene 24, 2433–2440 (2005).
Peng, J. et al. A proteomics approach to understanding protein ubiquitination. Nature Biotechnol. 21, 921–926 (2003).
Kobayashi, Y. et al. SIRT1 is critical regulator of FOXO-mediated transcription in response to oxidative stress. Int. J. Mol. Med. 16, 237–243 (2005).
Greer, E. L. & Brunet, A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene 24, 7410–7425 (2005).
Sigismund, S., Polo, S. & Di Fiore, P. P. Signaling through monoubiquitination. Curr. Top. Microbiol. Immunol. 286, 149–185 (2004).
Dikic, I. Mechanisms controlling EGF receptor endocytosis and degradation. Biochem. Soc. Trans. 31, 1178–1181 (2003).
Fang, D. & Kerppola, T. K. Ubiquitin-mediated fluorescence complementation reveals that Jun ubiquitinated by Itch/AIP4 is localized to lysosomes. Proc. Natl Acad. Sci. USA 101, 14782–14787 (2004).
Haglund, K. et al. Multiple monoubiquitination of RTKs is sufficient for their endocytosis and degradation. Nature Cell Biol. 5, 461–466 (2003).
Furuyama, T., Nakazawa, T., Nakano, I. & Mori, N. Identification of the differential distribution patterns of mRNAs and consensus binding sequences for mouse DAF-16 homologues. Biochem. J. 349, 629–634 (2000).
Colland, F. et al. Functional proteomics mapping of a human signaling pathway. Genome Res. 14, 1324–1332 (2004).
Li, M. et al. Deubiquitination of p53 by HAUSP is an important pathway for p53 stabilization. Nature 416, 648–653 (2002).
Li, M., Brooks, C. L., Kon, N. & Gu, W. A dynamic role of HAUSP in the p53-Mdm2 pathway. Mol. Cell 13, 879–886 (2004).
Meulmeester, E. et al. Loss of HAUSP-mediated deubiquitination contributes to DNA damage-induced destabilization of Hdmx and Hdm2. Mol. Cell 18, 565–576 (2005).
van der Knaap, J. A. et al. GMP synthetase stimulates histone H2B deubiquitylation by the epigenetic silencer USP7. Mol. Cell 17, 695–707 (2005).
Amerik, A. Y. & Hochstrasser, M. Mechanism and function of deubiquitinating enzymes. Biochim. Biophys. Acta 1695, 189–207 (2004).
Yin, L., Krantz, B., Russell, N. S., Deshpande, S. & Wilkinson, K. D. Nonhydrolyzable diubiquitin analogues are inhibitors of ubiquitin conjugation and deconjugation. Biochemistry 39, 10001–10010 (2000).
Borodovsky, A. et al. Chemistry-based functional proteomics reveals novel members of the deubiquitinating enzyme family. Chem. Biol. 9, 1149–1159 (2002).
Medema, R. H., Kops, G. J., Bos, J. L. & Burgering, B. M. AFX-like Forkhead transcription factors mediate cell-cycle regulation by Ras and PKB through p27kip1. Nature 404, 782–787 (2000).
Kops, G. J. et al. Control of cell cycle exit and entry by protein kinase B-regulated forkhead transcription factors. Mol. Cell. Biol. 22, 2025–2036 (2002).
Mamillapalli, R. et al. PTEN regulates the ubiquitin-dependent degradation of the CDK inhibitor p27(KIP1) through the ubiquitin E3 ligase SCF(SKP2). Curr. Biol. 11, 263–267 (2001).
Luo, J. et al. Negative control of p53 by Sir2α promotes cell survival under stress. Cell 107, 137–148 (2001).
Vaziri, H. et al. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell 107, 149–159 (2001).
Burgering, B. M. & Coffer, P. J. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature 376, 599–602 (1995).
Medema, R. H., Herrera, R. E., Lam, F. & Weinberg, R. A. Growth suppression by p16ink4 requires functional retinoblastoma protein. Proc. Natl Acad. Sci. USA 92, 6289–6293 (1995).
Acknowledgements
We thank J.L. Bos and L. Price for critically reading the manuscript; the members of the Bos and Burgering labs for helpful discussions; T. Kerppola., J. Rodriguez, A. Borodovsky and D. Bohmann for kindly providing DNA constructs; and B. Kumar and J. van der Knaap for supplying purified USP7. The authors also acknowledge H. Ovaa for providing the HA–ubiquitin probe and Hybrigenics staff for their contributions. This work was supported by grants from the Dutch Cancer Society (KWF, UU-2001-2438) to A.v.d.H.; from The Netherlands organization for scientific research (NWO, Zon-MW 906-02-041) to M.M.M.; from the Cancer Genomics center to N.v.d.B.; and from the European Commission Transfog Consortium (LSHC-CT-2004-503438) to A.B.B.
Author information
Authors and Affiliations
Contributions
A.v.d.H., A.M.M.d.V-S. and B.M.T.B. conceived and designed the expermiments. A.v.d.H., A.M.M.d.V-S., A.B.B., M.H.v.T. and N.v.d.B performed the experiments. F.C. and M.M.M. contributed essential materials. A.v.d.H., A.M.M.d.V-S. and B.M.T.B analysed the data. A.v.d.H. and B.M.T.B wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplemantary Figures S1, S2, S3, S4, S5 and S6 (PDF 5465 kb)
Rights and permissions
About this article
Cite this article
van der Horst, A., de Vries-Smits, A., Brenkman, A. et al. FOXO4 transcriptional activity is regulated by monoubiquitination and USP7/HAUSP. Nat Cell Biol 8, 1064–1073 (2006). https://doi.org/10.1038/ncb1469
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1469
This article is cited by
-
FOXO transcription factors as mediators of stress adaptation
Nature Reviews Molecular Cell Biology (2024)
-
Angiotensin-converting enzyme inhibitor promotes angiogenesis through Sp1/Sp3-mediated inhibition of notch signaling in male mice
Nature Communications (2023)
-
Prediction of human protein interactome of dengue virus non-structural protein 5 (NS5) and its downstream immunological implications
3 Biotech (2023)
-
USP7 targets XIAP for cancer progression: Establishment of a p53-independent therapeutic avenue for glioma
Oncogene (2022)
-
Therapeutic strategies targeting FOXO transcription factors
Nature Reviews Drug Discovery (2021)