Abstract
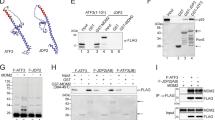
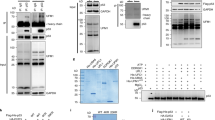
The tumour suppressor p53 induces apoptosis or cell-cycle arrest in response to genotoxic and other stresses1,2. In unstressed cells, the anti-proliferative effects of p53 are restrained by mouse double minute 2 (Mdm2), a ubiquitin ligase (E3) that promotes p53 ubiquitination and degradation3. Mdm2 also mediates its own degradation through auto-ubiquitination. It is unclear how the cis- and trans-E3 activities of Mdm2, which have opposing effects on cell fate, are differentially regulated. Here, we show that death domain-associated protein (Daxx)4 is required for Mdm2 stability. Downregulation of Daxx decreases Mdm2 levels, whereas overexpression of Daxx strongly stabilizes Mdm2. Daxx simultaneously binds to Mdm2 and the deubiquitinase Hausp, and it mediates the stabilizing effect of Hausp on Mdm2. In addition, Daxx enhances the intrinsic E3 activity of Mdm2 towards p53. On DNA damage, Daxx dissociates from Mdm2, which correlates with Mdm2 self-degradation. These findings reveal that Daxx modulates the function of Mdm2 at multiple levels and suggest that the disruption of the Mdm2–Daxx interaction may be important for p53 activation in response to DNA damage.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Vogelstein, B., Lane, D. & Levine, A. J. Surfing the p53 network. Nature 408, 307–310 (2000).
Vousden, K. H. & Lu, X. Live or let die: the cell's response to p53. Nature Rev. Cancer 2, 594–604 (2002).
Michael, D. & Oren, M. The p53–Mdm2 module and the ubiquitin system. Semin. Cancer Biol. 13, 49–58 (2003).
Yang, X., Khosravi-Far, R., Chang, H. Y. & Baltimore, D. Daxx, a novel Fas-binding protein that activates JNK and apoptosis. Cell 89, 1067–1076 (1997).
Chang, H. Y., Nishitoh, H., Yang, X., Ichijo, H. & Baltimore, D. Activation of apoptosis signal-regulating kinase 1 (ASK1) by the adapter protein Daxx. Science 281, 1860–1863 (1998).
Perlman, R., Schiemann, W. P., Brooks, M. W., Lodish, H. F. & Weinberg, R. A. TGF-β-induced apoptosis is mediated by the adapter protein Daxx that facilitates JNK activation. Nature Cell Biol. 3, 708–714 (2001).
Zhong, S. et al. Promyelocytic leukemia protein (PML) and Daxx participate in a novel nuclear pathway for apoptosis. J. Exp. Med. 191, 631–640 (2000).
Salomoni, P. & Khelifi, A. F. Daxx: death or survival protein? Trends Cell Biol. 16 97–104 (2006).
Michaelson, J. S., Bader, D., Kuo, F., Kozak, C. & Leder, P. Loss of Daxx, a promiscuously interacting protein, results in extensive apoptosis in early mouse development. Genes Dev. 13, 1918–1923 (1999).
Zhao, L. Y. et al. Negative regulation of p53 functions by Daxx and the involvement of MDM2. J. Biol. Chem. 279, 50566–50579 (2004).
Gostissa, M. et al. The transcriptional repressor hDaxx potentiates p53-dependent apoptosis. J. Biol. Chem. 279, 48013–48023 (2004).
Kim, E. J., Park, J. S. & Um, S. J. Identification of Daxx interacting with p73, one of the p53 family, and its regulation of p53 activity by competitive interaction with PML. Nucleic Acids Res. 31, 5356–5367 (2003).
Fakharzadeh, S. S., Trusko, S. P. & George, D. L. Tumorigenic potential associated with enhanced expression of a gene that is amplified in a mouse tumor cell line. EMBO J. 10, 1565–1569 (1991).
Haupt, Y., Maya, R., Kazaz, A. & Oren, M. Mdm2 promotes the rapid degradation of p53. Nature 387, 296–299 (1997).
Kubbutat, M. H., Jones, S. N. & Vousden, K. H. Regulation of p53 stability by Mdm2. Nature 387, 299–303 (1997).
Fuchs, S. Y., Adler, V., Buschmann, T., Wu, X. & Ronai, Z. Mdm2 association with p53 targets its ubiquitination. Oncogene 17, 2543–2547 (1998).
Honda, R., Tanaka, H. & Yasuda, H. Oncoprotein MDM2 is a ubiquitin ligase E3 for tumor suppressor p53. FEBS Lett. 420, 25–27 (1997).
Fang, S., Jensen, J. P., Ludwig, R. L., Vousden, K. H. & Weissman, A. M. Mdm2 is a RING finger-dependent ubiquitin protein ligase for itself and p53. J. Biol. Chem. 275, 8945–8951 (2000).
Honda, R. & Yasuda, H. Activity of MDM2, a ubiquitin ligase, toward p53 or itself is dependent on the RING finger domain of the ligase. Oncogene 19, 1473–1476 (2000).
Bunz, F. et al. Disruption of p53 in human cancer cells alters the responses to therapeutic agents. J. Clin. Invest. 104, 263–269 (1999).
Tang, J. et al. A novel transcription regulatory complex containing death domain-associated protein and the ATR-X syndrome protein. J. Biol. Chem. 279, 20369–20377 (2004).
Everett, R. D. et al. A novel ubiquitin-specific protease is dynamically associated with the PML nuclear domain and binds to a herpesvirus regulatory protein. EMBO J. 16, 1519–1530 (1997).
Li, M. et al. Deubiquitination of p53 by HAUSP is an important pathway for p53 stabilization. Nature 416, 648–653 (2002).
Cummins, J. M. et al. Tumour suppression: disruption of HAUSP gene stabilizes p53. Nature 428, doi: 10.1038/nature02501 (2004).
Li, M., Brooks, C. L., Kon, N. & Gu, W. A dynamic role of HAUSP in the p53-Mdm2 pathway. Mol. Cell 13, 879–886 (2004).
Stommel, J. M. & Wahl, G. M. Accelerated MDM2 auto-degradation induced by DNA-damage kinases is required for p53 activation. EMBO J. 23, 1547–1556 (2004).
Meulmeester, E. et al. Loss of HAUSP-mediated deubiquitination contributes to DNA damage-induced destabilization of Hdmx and Hdm2. Mol. Cell 18, 565–576 (2005).
Maya, R. et al. ATM-dependent phosphorylation of Mdm2 on serine 395: role in p53 activation by DNA damage. Genes Dev. 15, 1067–1077 (2001).
Momand, J., Jung, D., Wilczynski, S. & Niland, J. The MDM2 gene amplification database. Nucleic Acids Res. 26, 3453–3459 (1998).
Acknowledgements
We thank P. Leder for wild type and Daxx−/− ES cells, H. Wu and Y. Xiong for p53−/− Mdm2−/− MEF cells, W. Gu for Hausp cDNA, G. Maul and B. Vogelstein for reagents, and D. George and S. Fuchs for advice. We also thank the Proteomic Core Facility of the Abramson Cancer Center at the University of Pennsylvania for mass spectrometry analysis and the National Cell Culture Center for providing HeLa S3 cells. J.T. was a postdoctoral appointee of an National Cancer Institute training grant (T32CA09140). X.Y. is supported by National Institutes of Health (NIH) grants (CA88868 and GM60911) and a Leukemia & Lymphoma Society Scholar Award.
Author information
Authors and Affiliations
Contributions
J.T. L.Q. And X.Y conceived and designed the experiments, analysed data and wrote the paper. J.T. and L.Q. performed the experiments. J.Z. and J.M. performed ES cell culture. W.W. and W.E. contributed reagents and performed apoptosis analysis. Y.Y.D. performed real-time RT−PCR.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures S1, S2, S3, S4 and S5 (PDF 1023 kb)
Rights and permissions
About this article
Cite this article
Tang, J., Qu, LK., Zhang, J. et al. Critical role for Daxx in regulating Mdm2. Nat Cell Biol 8, 855–862 (2006). https://doi.org/10.1038/ncb1442
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1442
This article is cited by
-
HIRA vs. DAXX: the two axes shaping the histone H3.3 landscape
Experimental & Molecular Medicine (2024)
-
S100A6 inhibits MDM2 to suppress breast cancer growth and enhance sensitivity to chemotherapy
Breast Cancer Research (2023)
-
DAXX-ATRX regulation of p53 chromatin binding and DNA damage response
Nature Communications (2022)
-
Reciprocal regulation of Daxx and PIK3CA promotes colorectal cancer cell growth
Cellular and Molecular Life Sciences (2022)
-
TRIM15 and CYLD regulate ERK activation via lysine-63-linked polyubiquitination
Nature Cell Biology (2021)