Abstract
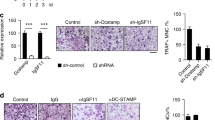
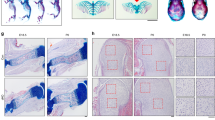
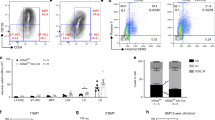
Semaphorins and their receptors have diverse functions in axon guidance, organogenesis, vascularization and/or angiogenesis, oncogenesis and regulation of immune responses1,2,3,4,5,6,7,8,9,10,11. The primary receptors for semaphorins are members of the plexin family2,12,13,14. In particular, plexin-A1, together with ligand-binding neuropilins, transduces repulsive axon guidance signals for soluble class III semaphorins15, whereas plexin-A1 has multiple functions in chick cardiogenesis as a receptor for the transmembrane semaphorin, Sema6D, independent of neuropilins16. Additionally, plexin-A1 has been implicated in dendritic cell function in the immune system17. However, the role of plexin-A1 in vivo, and the mechanisms underlying its pleiotropic functions, remain unclear. Here, we generated plexin-A1-deficient (plexin-A1−/−) mice and identified its important roles, not only in immune responses, but also in bone homeostasis. Furthermore, we show that plexin-A1 associates with the triggering receptor expressed on myeloid cells-2 (Trem-2), linking semaphorin-signalling to the immuno-receptor tyrosine-based activation motif (ITAM)-bearing adaptor protein, DAP12. These findings reveal an unexpected role for plexin-A1 and present a novel signalling mechanism for exerting the pleiotropic functions of semaphorins.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Tessier-Lavigne, M. & Goodman, S. C. The molecular biology of axon guidance. Science 274, 1123–1133 (1996).
Pasterkamp, R. J. & Kolodkin, A. L. Semaphorin junction: making tracks toward neural connectivity. Curr. Opin. Neurobiol. 13, 79–89 (2003).
Sekido, Y. et al. Human semaphorins A(V) and IV reside in the 3p21.3 small cell lung cancer deletion region and demonstrate distinct expression patterns. Proc. Natl Acad. Sci. USA 93, 4120–4125 (1996).
Gu, C. et al. Neuropilin-1 conveys semaphorin and VEGF signaling during neural and cardiovascular development. Dev. Cell 5, 45–57 (2003).
Toyofuku, T. et al. Guidance of myocardial patterning in cardiac development by Sema6D reverse signalling. Nature Cell Biol. 6, 1204–1211 (2004).
Kumanogoh, A. et al. Identification of CD72 as a lymphocyte receptor for the class IV semaphorin CD100: a novel mechanism for regulating B cell signaling. Immunity 13, 621–631 (2000).
Shi, W. et al. The class IV semaphorin CD100 plays nonredundant roles in the immune system: defective B and T cell activation in CD100-deficient mice. Immunity 13, 633–642 (2000).
Kumanogoh, A. et al. Class IV semaphorin Sema4A enhances T-cell activation and interacts with Tim-2. Nature 419, 629–633 (2002).
Kumanogoh, A. et al. Nonredundant roles of Sema4A in the immune system: defective T cell priming and Th1/Th2 regulation in Sema4A-deficient mice. Immunity 22, 305–316 (2005).
Kikutani, H. & Kumanogoh, A. Semaphorins in interactions between T cells and antigen-presenting cells. Nature Rev. Immunol. 3, 159–167 (2003).
Elhabazi, A., Marie-Cardine, A., Chabbert- de Ponnat, I., Bensussan, A. & Boumsell, L. Structure and function of the immune semaphorin CD100/SEMA4D. Crit. Rev. Immunol. 23, 65–81 (2003).
Tamagnone, L. & Comoglio, P. M. Signalling by semaphorin receptors: cell guidance and beyond. Trends Cell Biol. 10, 377–383 (2000).
Granziero, L. et al. CD100/Plexin-B1 interactions sustain proliferation and survival of normal and leukemic CD5+ B lymphocytes. Blood 101, 1962–1969 (2003).
Walzer, T., Galibert, L., Comeau, M. R. & De Smedt, T. Plexin C1 engagement on mouse dendritic cells by viral semaphorin A39R induces actin cytoskeleton rearrangement and inhibits integrin-mediated adhesion and chemokine-induced migration. J. Immunol. 174, 51–59 (2005).
Takahashi, T. et al. Plexin–neuropilin-1 complexes form functional semaphorin-3A receptors. Cell 99, 59–69 (1999).
Toyofuku, T. et al. Dual roles of Sema6D in cardiac morphogenesis through region-specific association of its receptor, Plexin-A1, with off-track and vascular endothelial growth factor receptor type 2. Genes Dev. 18, 435–447 (2004).
Wong, A. W. et al. CIITA-regulated plexin-A1 affects T-cell-dendritic cell interactions. Nature Immunol. 4, 891–898 (2003).
Lacey, D. L. et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93, 165–176 (1998).
Theill, L. E., Boyle, W. J. & Penninger, J. M. RANK-L and RANK: T cells, bone loss, and mammalian evolution. Annu. Rev. Immunol. 20, 795–823 (2002).
Takahashi, T. & Strittmatter, S. M. Plexina1 autoinhibition by the plexin sema domain. Neuron 29, 429–439 (2001).
Giordano, S. et al. The semaphorin 4D receptor controls invasive growth by coupling with Met. Nature Cell Biol. 4, 720–724 (2002).
Lanier, L. L. & Bakker, A. B. The ITAM-bearing transmembrane adaptor DAP12 in lymphoid and myeloid cell function. Immunol. Today 21, 611–614 (2000).
Colonna, M. TREMs in the immune system and beyond. Nature Rev. Immunol. 3, 445–453 (2003).
Kaifu, T. et al. Osteopetrosis and thalamic hypomyelinosis with synaptic degeneration in DAP12-deficient mice. J. Clin. Invest. 111, 323–332 (2003).
Koga, T. et al. Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature 428, 758–763 (2004).
Bakker, A. B. et al. DAP12-deficient mice fail to develop autoimmunity due to impaired antigen priming. Immunity 13, 345–353 (2000).
Paloneva, J. et al. Loss-of-function mutations in TYROBP (DAP 12) result in a presenile dementia with bone cysts. Nature Genet. 25, 357–361 (2000).
Paloneva, J. et al. Mutations in two genes encoding different subunits of a receptor signaling complex result in an identical disease phenotype. Am. J. Hum. Genet. 71, 656–662 (2002).
Turner, L. J., Nicholls, S. & Hall, A. The activity of the plexin-A1 receptor is regulated by Rac. J. Biol. Chem. 279, 33199–33205 (2004).
Barnden, M. J., Allison, J., Heath, W. R. & Carbone, F. R. Defective TCR expression in transgenic mice constructed using cDNA-based α- and β-chain genes under the control of heterologous regulatory elements. Immunol. Cell Biol. 76, 34–40 (1998).
Acknowledgements
We thank K. Kubota for excellent secretarial assistance. We are grateful to H. Murayama and S. Sato for critical discussion and excellent bone analysis, including microCT and histology. We are also grateful to N. Yamamoto, H. Takayanagi, T. Nakano, I. Ishikawa, H. Arase, N. Yamada, T. Kaisho and K. Hoshino for providing materials, critical advice, discussion and encouragement. We also thank T. Yazawa, A. Kawai, J. Yoshida, K Shiozaki, N. Okita, N. Iwami and K. Nakamura for technical support. This study was supported by research grants from the Ministry of Education, Culture, Sports, Science and Technology, Japan and from the Core Research for Evolutional Science and Technology (CREST) program of the Japanese Science and Technology Agency (JST) to A.K. and H.K.
Author information
Authors and Affiliations
Contributions
N.T. performed the main experimental work and data analysis for bone analysis. H.T. performed the main experimental work and data analysis for the immune responses. T.T., T.T., T.O., K.Y., M.M., M.Y., D.V.R.P., K.S. and S.S. also performed experimental work. M.I. performed calcium signalling analysis. S.A., K.T., M.I. and M.O. were involved in generating knockout mice. M.I. and T.T. performed the DAP12 analysis. A.K. and H.K. co-organized and performed project planning, data analysis, discussion and writing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures S1, S2, S3, S4, S5 and S6 (PDF 653 kb)
Supplementary Information
Supplementary Movie S1 (AVI 2476 kb)
Supplementary Information
Supplementary Movie S2 (AVI 2476 kb)
Rights and permissions
About this article
Cite this article
Takegahara, N., Takamatsu, H., Toyofuku, T. et al. Plexin-A1 and its interaction with DAP12 in immune responses and bone homeostasis. Nat Cell Biol 8, 615–622 (2006). https://doi.org/10.1038/ncb1416
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1416
This article is cited by
-
MiR-148a deletion protects from bone loss in physiological and estrogen-deficient mice by targeting NRP1
Cell Death Discovery (2022)
-
Semaphorin 3A regulates alveolar bone remodeling on orthodontic tooth movement
Scientific Reports (2022)
-
Semaphorin3A increases M1-like microglia and retinal ganglion cell apoptosis after optic nerve injury
Cell & Bioscience (2021)
-
Neuroimmune semaphorins as costimulatory molecules and beyond
Molecular Medicine (2018)
-
The role of semaphorins in immune responses and autoimmune rheumatic diseases
Nature Reviews Rheumatology (2018)