Abstract
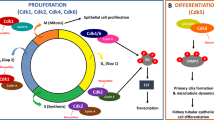
Autosomal-dominant polycystic kidney disease (ADPKD) is the most common hereditary kidney disease and is characterized by progressive cyst formation and ultimate loss of renal function. Increased cell proliferation is a key feature of the disease. Here, we show that the ADPKD protein polycystin-2 (PC2) regulates the cell cycle through direct interaction with Id2, a member of the helix–loop–helix (HLH) protein family that is known to regulate cell proliferation and differentiation. Id2 expression suppresses the induction of a cyclin-dependent kinase inhibitor, p21, by either polycystin-1 (PC1) or PC2. The PC2–Id2 interaction is regulated by PC1-dependent phosphorylation of PC2. Enhanced Id2 nuclear localization is seen in human and mouse cystic kidneys. Inhibition of Id2 expression by RNA interference corrects the hyperproliferative phenotype of PC1 mutant cells. We propose that Id2 has a crucial role in cell-cycle regulation that is mediated by PC1 and PC2.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Gabow, P. A. Autosomal dominant polycystic kidney disease. Am. J. Kidney Dis. 22, 511–512 (1993).
Nadasdy, T. et al. Proliferative activity of cyst epithelium in human renal cystic diseases. J. Am. Soc. Nephrol. 5, 1462–1468 (1995).
Grantham, J. J. Homer Smith Award. Fluid secretion, cellular proliferation, and the pathogenesis of renal epithelial cysts. J. Am. Soc. Nephrol. 3, 1841–1857 (1993).
Grantham, J. J. Polycystic kidney disease: from the bedside to the gene and back. Curr. Opin. Nephrol. Hypertens. 10, 533–542 (2001).
Calvet, J. P. Injury and development in polycystic kidney disease. Curr. Opin. Nephrol. Hypertens. 3, 340–348 (1994).
Wilson, P. D. Epithelial cell polarity and disease. Am. J. Physiol. 272, F434–F442 (1997).
The International Polycystic Kidney Disease Consortium. Polycystic kidney disease: the complete structure of the PKD1 gene and its protein. Cell 81, 289–298 (1995).
Hughes, J. et al. The polycystic kidney disease 1 (PKD1) gene encodes a novel protein with multiple cell recognition domains. Nature Genet. 10, 151–160 (1995).
Delmas, P. et al. Constitutive activation of G-proteins by polycystin-1 is antagonized by polycystin-2. J. Biol. Chem. 277, 11276–11283 (2002).
Parnell, S. C. et al. Polycystin-1 activation of c-Jun N-terminal kinase and AP-1 is mediated by heterotrimeric G proteins. J. Biol. Chem. 277, 19566–71952 (2002).
Stayner, C. & Zhou, J. Polycystin channels and kidney disease. Trends Pharmacol. Sci. 22, 543–546 (2001).
Vassilev, P. M. et al. Polycystin-2 is a novel cation channel implicated in defective intracellular Ca(2+) homeostasis in polycystic kidney disease. Biochem. Biophys. Res. Commun. 282, 341–350 (2001).
Gonzalez-Perrett, S. et al. Polycystin-2, the protein mutated in autosomal dominant polycystic kidney disease (ADPKD), is a Ca2+-permeable nonselective cation channel. Proc. Natl Acad. Sci. USA 98, 1182–1187 (2001).
Koulen, P. et al. Polycystin-2 is an intracellular calcium release channel. Nature Cell Biol. 4, 191–197 (2002).
Qian, F. et al. PKD1 interacts with PKD2 through a probable coiled-coil domain. Nature Genet. 16, 179–183 (1997).
Tsiokas, L., Kim, E., Arnould, T., Sukhatme, V. P. & Walz, G. Homo- and heterodimeric interactions between the gene products of PKD1 and PKD2. Proc. Natl Acad. Sci. USA 94, 6965–6970 (1997).
Lakkis, M. & Zhou, J. Molecular complexes formed with polycystins. Nephron 93, E3–E8 (2003).
Li, Q. et al. Polycystin-2 associates with tropomyosin-1, an actin microfilament component. J. Mol. Biol. 325, 949–962 (2003).
Li, Q., Shen, P. Y., Wu, G. & Chen, X. Z. Polycystin-2 interacts with troponin I, an angiogenesis inhibitor. Biochemistry 42, 450–457 (2003).
Luo, Y., Vassilev, P. M., Li, X., Kawanabe, Y. & Zhou, J. Native polycystin 2 functions as a plasma membrane Ca2+-permeable cation channel in renal epithelia. Mol. Cell. Biol. 23, 2600–2607 (2003).
Nauli, S. M. et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nature Genet. 33, 129–137 (2003).
Lu, W. et al. Perinatal lethality with kidney and pancreas defects in mice with a targeted Pkd1 mutation. Nature Genet 17, 179–181 (1997).
Lu, W. et al. Comparison of Pkd1-targeted mutants reveals that loss of polycystin-1 causes cystogenesis and bone defects. Hum. Mol. Genet. 10, 2385–2396 (2001).
Wu, G. et al. Somatic inactivation of Pkd2 results in polycystic kidney disease. Cell 93, 177–188 (1998).
Delmas, P. et al. Gating of the polycystin ion channel signaling complex in neurons and kidney cells. FASEB J. 18, 740–742 (2004).
Murre, C. et al. Interactions between heterologous helix-loop-helix proteins generate complexes that bind specifically to a common DNA sequence. Cell 58, 537–544 (1989).
Prabhu, S., Ignatova, A., Park, S. T. & Sun, X. H. Regulation of the expression of cyclin-dependent kinase inhibitor p21 by E2A and Id proteins. Mol. Cell. Biol. 17, 5888–5896 (1997).
Peverali, F. A. et al. Regulation of G1 progression by E2A and Id helix-loop-helix proteins. EMBO J. 13, 4291–4301 (1994).
Pagliuca, A., Gallo, P., De Luca, P. & Lania, L. Class A helix-loop-helix proteins are positive regulators of several cyclin-dependent kinase inhibitors' promoter activity and negatively affect cell growth. Cancer Res. 60, 1376–1382 (2000).
Fields, S. & Song, O. A novel genetic system to detect protein-protein interactions. Nature 340, 245–246 (1989).
Kim, E. et al. The polycystic kidney disease 1 gene product modulates Wnt signaling. J. Biol. Chem. 274, 4947–4953 (1999).
Bhunia, A. K. et al. PKD1 induces p21(waf1) and regulation of the cell cycle via direct activation of the JAK-STAT signaling pathway in a process requiring PKD2. Cell 109, 157–168 (2002).
Hara, E., Hall, M. & Peters, G. Cdk2-dependent phosphorylation of Id2 modulates activity of E2A-related transcription factors. EMBO J. 16, 332–342 (1997).
Matsumura, M. E., Lobe, D. R. & McNamara, C. A. Contribution of the helix-loop-helix factor Id2 to regulation of vascular smooth muscle cell proliferation. J. Biol. Chem. 277, 7293–7297 (2002).
Trudel, M., Barisoni, L., Lanoix, J. & D'Agati, V. Polycystic kidney disease in SBM transgenic mice: role of c-myc in disease induction and progression. Am. J. Pathol. 152, 219–229 (1998).
Reeders, S. T. Multilocus polycystic disease. Nature Genet. 1, 235–237 (1992).
Qian, F., Watnick, T. J., Onuchic, L. F. & Germino, G. G. The molecular basis of focal cyst formation in human autosomal dominant polycystic kidney disease type I. Cell 87, 979–987 (1996).
Rockman, S. P. et al. Id2 is a target of the β-catenin/T cell factor pathway in colon carcinoma. J. Biol. Chem. 276, 45113–45119 (2001).
Kondo, M. et al. A role for Id in the regulation of TGF-β-induced epithelial-mesenchymal transdifferentiation. Cell Death Differ. 11, 1092–1101 (2004).
Lasorella, A., Noseda, M., Beyna, M., Yokota, Y. & Iavarone, A. Id2 is a retinoblastoma protein target and mediates signalling by Myc oncoproteins. Nature 407, 592–598 (2000).
He, T. C. et al. Identification of c-MYC as a target of the APC pathway. Science 281, 1509–1512 (1998).
Trudel, M. et al. C-myc-induced apoptosis in polycystic kidney disease is Bcl-2 and p53 independent. J. Exp. Med. 186, 1873–1884 (1997).
Deed, R. W., Jasiok, M. & Norton, J. D. Attenuated function of a variant form of the helix-loop-helix protein, Id-3, generated by an alternative splicing mechanism. FEBS Lett. 393, 113–116 (1996).
Kowanetz, M., Valcourt, U., Bergstrom, R., Heldin, C. H. & Moustakas, A. Id2 and Id3 define the potency of cell proliferation and differentiation responses to transforming growth factor β and bone morphogenetic protein. Mol. Cell. Biol. 24, 4241–4254 (2004).
Kurooka, H. & Yokota, Y. Nucleo-cytoplasmic shuttling of Id2, a negative regulator of basic helix-loop-helix transcription factors. J. Biol. Chem. 280, 4313–4320 (2005).
Acknowledgements
We thank W.C. Forrester and J. Hasskarl for discussions; G. Sui, M.E. Matsumura, Q. Xi, P. Finnerty and A. Beck for technical assistance; V. Torres for some human tissues; and S. Somlo for the PC2-S812A construct. This work was supported by grants from the National Institutes of Health to J.Z. (DK53357 for PC2, DK40703 for PC1 and DK51050 for mouse work), and a Postdoctoral Fellowship from the Polycystic Kidney Disease Foundation (X.L.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary figure S1 (PDF 123 kb)
Rights and permissions
About this article
Cite this article
Li, X., Luo, Y., Starremans, P. et al. Polycystin-1 and polycystin-2 regulate the cell cycle through the helix–loop–helix inhibitor Id2. Nat Cell Biol 7, 1202–1212 (2005). https://doi.org/10.1038/ncb1326
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1326
This article is cited by
-
Lysine methyltransferase SMYD2 promotes triple negative breast cancer progression
Cell Death & Disease (2018)
-
Gαi-mediated TRPC4 activation by polycystin-1 contributes to endothelial function via STAT1 activation
Scientific Reports (2018)
-
The spectrum of autosomal dominant polycystic kidney disease in children and adolescents
Pediatric Nephrology (2017)
-
Role of apoptosis in the development of autosomal dominant polycystic kidney disease (ADPKD)
Cell and Tissue Research (2017)
-
The axoneme: the propulsive engine of spermatozoa and cilia and associated ciliopathies leading to infertility
Journal of Assisted Reproduction and Genetics (2016)