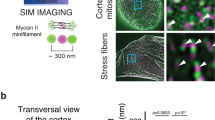
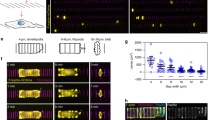
Abstract
The cell division axis determines the future positions of daughter cells and is therefore critical for cell fate. The positioning of the division axis has been mostly studied in systems such as embryos or yeasts, in which cell shape is well defined1,2. In these cases, cell shape anisotropy and cell polarity affect spindle orientation3,4,5. It remains unclear whether cell geometry or cortical cues are determinants for spindle orientation in mammalian cultured cells6,7. The cell environment is composed of an extracellular matrix (ECM), which is connected to the intracellular actin cytoskeleton via transmembrane proteins8. We used micro-contact printing to control the spatial distribution of the ECM on the substrate9 and demonstrated that it has a role in determining the orientation of the division axis of HeLa cells. On the basis of our analysis of the average distributions of actin-binding proteins in interphase and mitosis, we propose that the ECM controls the location of actin dynamics at the membrane, and thus the segregation of cortical components in interphase. This segregation is further maintained on the cortex of mitotic cells and used for spindle orientation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Palmer, R. E., Sullivan, D. S., Huffaker, T. & Koshland, D. Role of astral microtubules and actin in spindle orientation and migration in the budding yeast, Saccharomyces cerevisiae. J. Cell Biol. 119, 583–593 (1992).
Gonczy, P. Mechanisms of spindle positioning: focus on flies and worms. Trends Cell Biol. 12, 332–339 (2002).
Tsou, M. F., Ku, W., Hayashi, A. & Rose, L. S. PAR-dependent and geometry-dependent mechanisms of spindle positioning. J. Cell Biol. 160, 845–855 (2003).
Gray, D. et al. First cleavage of the mouse embryo responds to change in egg shape at fertilization. Curr. Biol. 14, 397–405 (2004).
Ahringer, J. Control of cell polarity and mitotic spindle positioning in animal cells. Curr. Opin. Cell Biol. 15, 73–81 (2003).
O'Connell, C. B. & Wang, Y. L. Mammalian spindle orientation and position respond to changes in cell shape in a dynein-dependent fashion. Mol. Biol. Cell 11, 1765–1774 (2000).
Reinsch, S. & Karsenti, E. Orientation of spindle axis and distribution of plasma membrane proteins during cell division in polarized MDCKII cells. J. Cell Biol. 126, 1509–1526 (1994).
Geiger, B., Bershadsky, A., Pankov, R. & Yamada, K. M. Transmembrane crosstalk between the extracellular matrix — cytoskeleton crosstalk. Nature Rev. Mol. Cell Biol. 2, 793–805 (2001).
Whitesides, G. M., Ostuni, E., Takayama, S., Jiang, X. & Ingber, D. E. Soft lithography in biology and biochemistry. Annu. Rev. Biomed. Eng. 3, 335–373 (2001).
Piel, M., Meyer, P., Khodjakov, A., Rieder, C. L. & Bornens, M. The respective contributions of the mother and daughter centrioles to centrosome activity and behavior in vertebrate cells. J. Cell Biol. 149, 317–330 (2000).
Hyman, A. A. & White, J. G. Determination of cell division axes in the early embryogenesis of Caenorhabditis elegans. J. Cell Biol. 105, 2123–2135 (1987).
Parker, K. et al. Directional control of lamellipodia extension by constraining cell shape and orienting cell tractional forces. FASEB J. 16, 1195–1204 (2002).
Weed, S. A. & Parsons, J. T. Cortactin: coupling membrane dynamics to cortical actin assembly. Oncogene 20, 6418–6434 (2001).
Louvet, S., Aghion, J., Santa-Maria, A., Mangeat, P. & Maro, B. Ezrin becomes restricted to outer cells following asymmetrical division in the preimplantation mouse embryo. Dev. Biol. 177, 568–579 (1996).
Fievet, B. T. et al. Phosphoinositide binding and phosphorylation act sequentially in the activation mechanism of ezrin. J. Cell Biol. 164, 653–659 (2004).
Bretscher, A., Edwards, K. & Fehon, R. G. ERM proteins and merlin: integrators at the cell cortex. Nature Rev. Mol. Cell Biol. 3, 586–599 (2002).
Mitchison, T. J. Actin based motility on retraction fibers in mitotic PtK2 cells. Cell Motil. Cytoskeleton 22, 135–151 (1992).
Angers-Loustau, A., Hering, R., Werbowetski, T. E., Kaplan, D. R. & Del Maestro, R. F. SRC regulates actin dynamics and invasion of malignant glial cells in three dimensions. Mol. Cancer Res. 2, 595–605 (2004).
Hanke, J. H. et al. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J. Biol. Chem. 271, 695–701 (1996).
Blake, R. A. et al. SU6656, a selective src family kinase inhibitor, used to probe growth factor signaling. Mol. Cell. Biol 20, 9018–9027 (2000).
DeMali, K. A., Barlow, C. A. & Burridge, K. Recruitment of the Arp2/3 complex to vinculin: coupling membrane protrusion to matrix adhesion. J. Cell Biol. 159, 881–891 (2002).
Tilghman, R. W. et al. Focal adhesion kinase is required for the spatial organization of the leading edge in migrating cells. J. Cell Sci. 118, 2613–2623 (2005).
Etienne-Manneville, S. & Hall, A. Cdc42 regulates GSK-3β and adenomatous polyposis coli to control cell polarity. Nature 421, 753–756 (2003).
Busson, S., Dujardin, D., Moreau, A., Dompierre, J. & De Mey, J. R. Dynein and dynactin are localized to astral microtubules and at cortical sites in mitotic epithelial cells. Curr. Biol. 8, 541–544 (1998).
Dujardin, D. L. et al. A role for cytoplasmic dynein and LIS1 in directed cell movement. J. Cell Biol. 163, 1205–1211 (2003).
Faulkner, N. E. et al. A role for the lissencephaly gene LIS1 in mitosis and cytoplasmic dynein function. Nature Cell Biol. 2, 784–791 (2000).
Wang, S. W., Griffin, F. J. & Clark, W. H., Jr. Cell-cell association directed mitotic spindle orientation in the early development of the marine shrimp Sicyonia ingentis. Development 124, 773–780 (1997).
Goldstein, B. Cell contacts orient some cell division axes in the Caenorhabditis elegans embryo. J. Cell Biol. 129, 1071–1080 (1995).
Pépin, A. & Chen, Y. in Alternative Lithography (ed. Sotomayor Torres, C. M.) 305–330 (Kluwer Academic/Plenum, Boston/Dordrecht/London, 2003).
Cuvelier, D., Rossier, O., Bassereau, P. & Nassoy, P. Micropatterned “adherent/repellent” glass surfaces for studying the spreading kinetics of individual red blood cells onto protein-decorated substrates. Eur. Biophys. J. 32, 342–354 (2003).
Acknowledgements
We would like to thank Y. Bellaiche and P. Chavrier for helpful discussions, D. E. Ingber for technical help during preliminary experiments, and M. Morgan and J. Sillibourne for carefully reading this manuscript. Part of this work was carried out in the clean room facility of the UMR168 at the Institut Curie. Supported by CNRS, Institut Curie and by HSFP, grant Ref RGP0064/2004 to M.B.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary figures S1, S2, S3 and S4 plus movies legends (PDF 627 kb)
Rights and permissions
About this article
Cite this article
Théry, M., Racine, V., Pépin, A. et al. The extracellular matrix guides the orientation of the cell division axis. Nat Cell Biol 7, 947–953 (2005). https://doi.org/10.1038/ncb1307
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1307
This article is cited by
-
Expression of genes regulating cell division in porcine follicular granulosa cells
Cell Division (2023)
-
In mitosis integrins reduce adhesion to extracellular matrix and strengthen adhesion to adjacent cells
Nature Communications (2023)
-
Mechanisms underlying spindle assembly and robustness
Nature Reviews Molecular Cell Biology (2023)
-
Fabrication of micro-nano patterned materials mimicking the topological structure of extracellular matrix for biomedical applications
Nano Research (2023)
-
CDK1–cyclin-B1-induced kindlin degradation drives focal adhesion disassembly at mitotic entry
Nature Cell Biology (2022)