Abstract
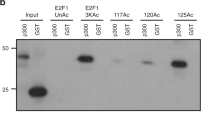
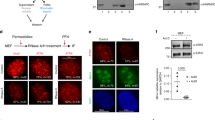
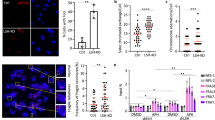
Here, we show a role for the RB1 family proteins in directing full heterochromatin formation. Mouse embryonic fibroblasts that are triply deficient for RB1 (retinoblastoma 1), RBL1 (retinoblastoma-like 1) and RBL2 (retinoblastoma-like 2) — known as TKO cells — show a marked genomic instability, which is coincidental with decreased DNA methylation, increased acetylation of histone H3 and decreased tri-methylation of histone H4 at lysine 20 (H4K20). Chromatin immunoprecipitation showed that H4K20 tri-methylation was specifically decreased at pericentric and telomeric chromatin. These defects are independent of E2F family function. Indeed, we show a direct interaction between the RB1 proteins and the H4K20 tri-methylating enzymes Suv4-20h1 and Suv4-20h2, indicating that the RB1 family has a role in controlling H4K20 tri-methylation by these histone methyltransferases. These observations indicate that the RB1 family is involved in maintaining overall chromatin structure and, in particular, that of constitutive heterochromatin, linking tumour suppression and the epigenetic definition of chromatin.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Lengauer, C., Kinzler, K. W. & Vogelstein, B. Genetic instabilities in human cancers. Nature 396, 643–649 (1998).
Harbour, J. W. & Dean, D. C. The RB1/E2F pathway: expanding roles and emerging paradigms. Genes Dev. 14, 2393–2409 (2000).
Sherr, C. J. & Weber, J. D. The ARF/p53 pathway. Curr. Opin. Genet. Dev. 10, 94–99 (2000).
Harbour, J. W. & Dean, D. C. Chromatin remodeling and RB1 activity. Curr. Opin. Cell Biol. 12, 685–689 (2000).
Robertson, K. D. et al. DNMT1 forms a complex with RB1, E2F1 and HDAC1 and represses transcription from E2F-responsive promoters. Nature Genet. 25, 338–342 (2000).
Luo, R. X., Postigo, A. A. & Dean, D. C. RB1 interacts with histone deacetylase to repress transcription. Cell 92, 463–473 (1998).
Nielsen, S. J. et al. RB1 targets histone H3 methylation and HP1 to promoters. Nature 412, 561–565 (2001).
Di Leonardo, A. et al. DNA rereplication in the presence of mitotic spindle inhibitors in human and mouse fibroblasts lacking either p53 or pRB1 function. Cancer Res. 57, 1013–1019 (1997).
Fukasawa, K., Choi, T., Kuriyama, R., Rulong, S. & Vande Woude, G. F. Abnormal centrosome amplification in the absence of p53. Science 271, 1744–1747 (1996).
Khan, S. H. & Wahl, G. M. p53 and pRB1 prevent rereplication in response to microtubule inhibitors by mediating a reversible G1 arrest. Cancer Res. 58, 396–401 (1998).
Sage, J. et al. Targeted disruption of the three RB1-related genes leads to loss of G(1) control and immortalization. Genes Dev. 14, 3037–3050 (2000).
Borel, F., Lohez, O. D., Lacroix, F. B. & Margolis, R. L. Multiple centrosomes arise from tetraploidy checkpoint failure and mitotic centrosome clusters in p53 and RB1 pocket protein-compromised cells. Proc. Natl Acad. Sci. USA 99, 9819–9824 (2002).
Dannenberg, J. H., van Rossum, A., Schuijff, L. & te Riele, H. Ablation of the retinoblastoma gene family deregulates G(1) control causing immortalization and increased cell turnover under growth-restricting conditions. Genes Dev. 14, 3051–3064 (2000).
Bernard, P. & Allshire, R. Centromeres become unstuck without heterochromatin. Trends Cell Biol. 12, 419–424 (2002).
Bosco, G., Du, W. & Orr-Weaver, T. L. DNA replication control through interaction of E2F-RB1 and the origin recognition complex. Nature Cell Biol. 3, 289–295 (2001).
Garcia-Cao, M., Gonzalo, S., Dean, D. & Blasco, M. A. A role for the RB1 family of proteins in controlling telomere length. Nature Genet. 32, 415–419 (2002).
Fraga, M. F. & Esteller, M. DNA methylation: a profile of methods and applications. Biotechniques 33, 632, 634, 636–649 (2002).
Lehnertz, B. et al. Suv39h-mediated histone H3 lysine 9 methylation directs DNA methylation to major satellite repeats at pericentric heterochromatin. Curr. Biol. 13, 1192–1200 (2003).
Schotta, G. et al. A silencing pathway to induce H3-K9 and H4-K20 tri-methylation at constitutive heterochromatin. Genes Dev. 18, 1251–1262 (2004).
Garcia-Cao, M., O'Sullivan, R., Peters, A. H., Jenuwein, T. & Blasco, M. A. Epigenetic regulation of telomere length in mammalian cells by the Suv39h1 and Suv39h2 histone methyltransferases. Nature Genet. 36, 94–99 (2004).
Peters, A. H. et al. Loss of the Suv39h histone methyltransferases impairs mammalian heterochromatin and genome stability. Cell 107, 323–337 (2001).
Kourmouli, N. et al. Heterochromatin and tri-methylated lysine 20 of histone 4 in mammals. J. Cell Sci. 117, 2491–2501 (2004).
Rowland, B.D. et al. E2F transcriptional repressor complexes are critical downstream targets of p19(ARF)/p53-induced proliferative arrest. Cancer Cell 2, 55–65 (2002).
Narita, M. et al. RB1-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell 113, 703–716 (2003).
Samper, E. et al. Normal telomere length and chromosomal end capping in poly(ADP-ribose) polymerase-deficient mice and primary cells despite increased chromosomal instability. J. Cell Biol. 154, 49–60 (2001).
Ballestar, E. et al. Methyl-CpG binding proteins identify novel sites of epigenetic inactivation in human cancer. EMBO J. 22, 6335–6345 (2003).
Herman, J. G., Graff, J. R., Myohanen, S., Nelkin, B. D. & Baylin, S. B. Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc. Natl Acad. Sci. USA 93, 9821–9826 (1996).
Lindner, H. et al. Separation of phosphorylated histone H1 variants by high-performance capillary electrophoresis. J. Chromatogr. 608, 211–216 (1992).
Goodwin, G. H., Nicolas, R. H. & Johns, E. W. A quantitative analysis of histone H1 in rabbit thymus nuclei. Biochem. J. 167, 485–488 (1977).
Gurley, L. R., Prentice, D. A., Valdez, J. G. & Spall, W. D. Histone fractionation by high-performance liquid chromatography on cyanoalkysilane (CN) reverse-phase columns. Anal. Biochem. 131, 465–477 (1983).
Palmero, I. & Serrano, M. Identification of the gene immediately downstream of the murine INK4a/ARF locus. Exp. Gerontol. 36, 1289–1302 (2001).
Acknowledgements
We thank A. Kohlmaier for help with the RT-PCR assays. We are very grateful to J. Sage and T. Jacks for the donation of numerous vials of DKO and TKO MEFs, as well as for their helpful advice. We are indebted to D. Peeper and W. Krek for providing the pBabePuro–E2F1-DB construct. We thank K. Wikenheiser, S. Wong and M. Serrano for advice and helpful comments. S.G. was supported by the National Institutes of Health and by the National Science Foundation; M.G.-C. by the Spanish Ministry of Science and Technology (MCYT); and R.E. by the Regional Government of Madrid (CAM). Research in the laboratory of T.J. is supported by the Institute of Molecular Pathology through Boehringer Ingelheim and by grants from the Vienna Economy Promotion Fund, the European Union and the GEN-AU initiative, which is financed by the Austrian Ministry of Education, Science and Culture. Research in M.A.B.'s laboratory is funded by the MCYT (SAF2001-1869, GEN2001-4856-C13-08), by the Regional Government of Madrid, CAM (08.1/0054/01), by the European Union (TELOSENS FIGH-CT.2002-00217, INTACT LSHC-CT-2003-506803, ZINCAGE FOOD-CT-2003-506850, RISC-RAD F16R-CT-2003-508842) and by the Josef Steiner Cancer Award 2003.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary figures S1, S2, S3 and S4 (PDF 774 kb)
Rights and permissions
About this article
Cite this article
Gonzalo, S., García-Cao, M., Fraga, M. et al. Role of the RB1 family in stabilizing histone methylation at constitutive heterochromatin. Nat Cell Biol 7, 420–428 (2005). https://doi.org/10.1038/ncb1235
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1235
This article is cited by
-
Cell cycle gene alterations associate with a redistribution of mutation risk across chromosomal domains in human cancers
Nature Cancer (2024)
-
Liquid–liquid phase separation in tumor biology
Signal Transduction and Targeted Therapy (2022)
-
Genome-wide DNA methylation patterns reveal clinically relevant predictive and prognostic subtypes in human osteosarcoma
Communications Biology (2022)
-
Epigenetics and beyond: targeting writers of protein lysine methylation to treat disease
Nature Reviews Drug Discovery (2021)
-
Integrated multi-omics analysis of RB-loss identifies widespread cellular programming and synthetic weaknesses
Communications Biology (2021)