Abstract
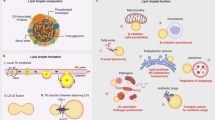
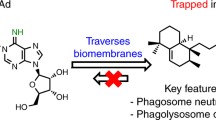
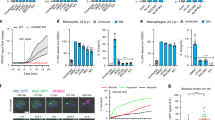
Pathogenic mycobacteria such as Mycobacterium tuberculosis and Mycobacterium avium facilitate disease by surviving intracellularly within a potentially hostile environment: the macrophage phagosome. They inhibit phagosome maturation processes, including fusion with lysosomes, acidification and, as shown here, membrane actin assembly. An in vitro assay developed for latex bead phagosomes (LBPs) provided insights into membrane signalling events that regulate phagosome actin assembly, a process linked to membrane fusion. Different lipids were found to stimulate or inhibit actin assembly by LBPs and mycobacterial phagosomes in vitro. In addition, selected lipids activated actin assembly and phagosome maturation in infected macrophages, resulting in a significant killing of M. tuberculosis and M. avium. In contrast, the polyunsaturated σ-3 lipids behaved differently and stimulated pathogen growth. Thus, lipids can be involved in both stimulatory and inhibitory signalling networks in the phagosomal membrane.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Barry, C.E. 3rd. Interpreting cell wall 'virulence factors' of Mycobacterium tuberculosis. Trends Microbiol. 9, 237–241 (2001).
Kaufmann, S.H. Is the development of a new tuberculosis vaccine possible? Nature Med. 6, 955–960 (2000).
Flynn, J.L. & Chan, J. Immunology of tuberculosis. Annu. Rev. Immunol. 19, 93–129 (2001).
Nathan, C. & Shiloh, M.U. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc. Natl Acad. Sci. USA 97, 8841–8848 (2000).
Vieira, O.V., Botelho, R.J. & Grinstein, S. Phagosome maturation: aging gracefully. Biochem. J. 366, 689–704 (2002).
Clemens, D.L. Characterization of the Mycobacterium tuberculosis phagosome. Trends Microbiol. 4, 113–118 (1996).
Russell, D.G. Mycobacterium tuberculosis: here today, and here tomorrow. Nature Rev. Mol. Cell Biol. 2, 569–577 (2001).
Haas, A. Reprogramming the phagocytic pathway–intracellular pathogens and their vacuoles. Mol. Membrane Biol. 15, 103–121 (1998).
Tjelle, T.E., Lovdal, T. & Berg, T. Phagosome dynamics and function. Bioessays 22, 255–263 (2000).
Fischer, K. et al. Mycobacterial lysocardiolipin is exported from phagosomes upon cleavage of cardiolipin by a macrophage-derived lysosomal phospholipase A2. J. Immunol. 167, 2187–2192 (2001).
Pieters, J. Entry and survival of pathogenic mycobacteria in macrophages. Microbes Infect. 3, 249–255 (2001).
Blackwell, J.M. et al. SLC11A1 (formerly NRAMP1) and disease resistance. Cell Microbiol. 3, 773–784 (2001).
Via, L.E. et al. Effects of cytokines on mycobacterial phagosome maturation. J. Cell Sci. 111, 897–905 (1998).
Akaki, T., Tomioka, H., Shimizu, T., Dekio, S. & Sato, K. Comparative roles of free fatty acids with reactive nitrogen intermediates and reactive oxygen intermediates in expression of the anti-microbial activity of macrophages against Mycobacterium tuberculosis. Clin. Exp. Immunol. 121, 302–310 (2000).
Defacque, H. et al. Involvement of ezrin/moesin in de novo actin assembly on phagosomal membranes. EMBO J. 19, 199–212 (2000).
Desjardins, M. et al. Molecular characterization of phagosomes. J. Biol. Chem. 269, 32194–32200 (1994).
Desjardins, M., Huber, L.A., Parton, R.G. & Griffiths, G. Biogenesis of phagolysosomes proceeds through a sequential series of interactions with the endocytic apparatus. J. Cell Biol. 24, 677–688 (1994).
Garin, J. et al. The phagosome proteome: insight into phagosome functions. J. Cell Biol. 152, 165–180 (2001).
Defacque, H. et al. Actin assembly induced by polylysine beads or purified phagosomes: quantitation by a new flow cytometry assay. Cytometry 41, 46–54 (2000).
Defacque, H. Phosphoinositides regulate membrane-dependent actin assembly by latex bead phagosomes. Mol. Biol. Cell 13, 1190–1202 (2002).
Emans, N., Nzala, N.N. & Desjardins, M. Protein phosphorylation during phagosome maturation. FEBS Lett. 398, 37–42 (1996).
Jahraus, A. et al. ATP-dependent membrane assembly of F-actin facilitates membrane fusion. Mol. Biol. Cell 12, 155–170 (2001).
Holowka, D. & Baird, B. Antigen-mediated IGE receptor aggregation and signaling: a window on cell surface structure and dynamics. Annu. Rev. Biophys. Biomol. Struct. 25, 79–112 (1996).
Simons, K. & Toomre, D. Lipid rafts and signal transduction. Nature Rev. Mol. Cell Biol. 1, 31–39 (2000).
Caroni, P. New EMBO members' review: actin cytoskeleton regulation through modulation of PI(4,5)P(2) rafts. EMBO J. 20, 4332–4336 (2001).
Rozelle, A.L. et al. Phosphatidylinositol 4,5-bisphosphate induces actin-based movement of raft-enriched vesicles through WASP–Arp2/3. Curr. Biol. 10, 311–320 (2000).
Dermine, J.F. et al. Flotillin-1-enriched lipid raft domains accumulate on maturing phagosomes. J. Biol. Chem. 276, 18507–18512 (2001).
Bornfeldt, K.E. et al. Sphingosine-1-phosphate inhibits PDGF-induced chemotaxis of human arterial smooth muscle cells: spatial and temporal modulation of PDGF chemotactic signal transduction. J. Cell Biol. 130, 193–206 (1995).
Spiegel, S. & Milstien, S. Sphingosine-1-phosphate: signaling inside and out. FEBS Lett. 476, 55–57 (2000).
Hannun, Y.A., Luberto, C. & Argraves, K.M. Enzymes of sphingolipid metabolism: from modular to integrative signaling. Biochemistry 40, 4893–4903 (2001).
Kuehnel, M.P. et al. Characterization of the intracellular survival of Mycobacterium avium ssp. paratuberculosis: phagosomal pH and fusogenicity in J774 macrophages compared with other mycobacteria. Cell Microbiol. 3, 551–566 (2001).
Guerin, I. & de Chastellier, C. Pathogenic mycobacteria disrupt the macrophage actin filament network. Infect. Immun. 68, 2655–2662 (2000).
Sturgill-Koszycki, S., Haddix, P.L. & Russell, D.G. The interaction between Mycobacterium and the macrophage analyzed by two-dimensional polyacrylamide gel electrophoresis. Electrophoresis 18, 2558–2565 (1997).
Hackam, D.J. et al. Regulation of phagosomal acidification. Differential targeting of Na+/H+ exchangers, Na+/K+-ATPases, and vacuolar-type H+-ATPases. J. Biol. Chem. 272, 29810–29820 (1997).
Sturgill-Koszycki, S. et al. Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science 263, 678–681 (1994).
Goni, F.M. & Alonso, A. Structure and functional properties of diacylglycerols in membranes. Prog. Lipid Res. 38, 1–48 (1999).
Paul, K.P. et al. Influence of n-6 and n-3 polyunsaturated fatty acids on the resistance to experimental tuberculosis. Metabolism 46, 619–624 (1997).
Chang, H.R. et al. Fish oil decreases natural resistance of mice to infection with Salmonella typhimurium. Metabolism 41, 1–2 (1992).
Kaplan, G.J., Fraser, R.I. & Comstock, G.W. Tuberculosis in Alaska. Am. Rev. Respir. Disease 105, 920–926 (1970).
Bang, H.O. & Dyerberg, J. Lipid metabolism and ischemic heart disease in Greenland Eskimos. Adv. Nutr. Res. 3, 1–22 (1980).
Snapper, S.B., Melton, R.E., Mustafa, S., Kieser, T. & Jacobs, W.R. Jr. Isolation and characterization of efficient plasmid transformation mutants of Mycobacterium smegmatis. Mol. Microbiol. 4, 1911–1199 (1990).
Acknowledgements
We wish to thank the following for their support and suggestions: C. Dennison, E. Elliott, H. Goodfellow, R. Goethe, A. Haas, R. Hodge, D. Holden, S. Kuznetsov, C. Roy, S. Trachtenberg, K. Unsworth, P. Valentin-Weigand and the members of the EMBL photolab, especially U. Ringeisen. E. Anes's work was supported by Fundaçâo para a Ciência e a Tecnologia (FCT) grant POCTI/38983/BCI/2001 with co-participation of the EU fund FEDER.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Anes, E., Kühnel, M., Bos, E. et al. Selected lipids activate phagosome actin assembly and maturation resulting in killing of pathogenic mycobacteria. Nat Cell Biol 5, 793–802 (2003). https://doi.org/10.1038/ncb1036
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1036
This article is cited by
-
Metabolomic changes in polyunsaturated fatty acids and eicosanoids as diagnostic biomarkers in Mycobacterium avium ssp. paratuberculosis (MAP)-inoculated Holstein–Friesian heifers
Veterinary Research (2022)
-
Comprehensive lipid and lipid-related gene investigations of host immune responses to characterize metabolism-centric biomarkers for pulmonary tuberculosis
Scientific Reports (2022)
-
Wirksamkeit von darmlöslichem Lecithin (Phosphatidylcholin) zur Behandlung der Colitis ulcerosa
MMW - Fortschritte der Medizin (2022)
-
Novel therapeutic evaluation biomarkers of lipid metabolism targets in uncomplicated pulmonary tuberculosis patients
Signal Transduction and Targeted Therapy (2021)
-
Performance of metabonomic serum analysis for diagnostics in paediatric tuberculosis
Scientific Reports (2020)