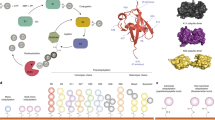
Abstract
Mass spectrometry (MS) analysis is applicable to a broad range of biological analytes and has the important advantage that it does not require analytes to be labeled. A drawback of MS methods, however, is the need for chromatographic steps to prepare the analyte, precluding MS from being used in chemical screening and rapid analysis. Here, we report that surfaces that are chemically tailored for characterization by matrix-assisted laser-desorption ionization time-of-flight MS eliminate the need for sample processing and make this technique adaptable to parallel screening experiments. The tailored substrates are based on self-assembled monolayers that present ligands that interact with target proteins and enzymes. We apply this method to screen a chemical library against protease activity of anthrax lethal factor, and report a compound that inhibits lethal factor activity with a Ki of 1.1 μM and blocks the cleavage of MEK1 in 293 cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Cacace, A., Banks, M., Spicer, T., Civoli, F. & Watson, J. An ultra-HTS process for the identification of small molecule modulators of orphan G-protein-coupled receptors. Drug Discov. Today 8, 785–792 (2003).
Khandurina, J. & Guttman, A. Microchip-based high-throughput screening analysis of combinatorial libraries. Curr. Opin. Chem. Biol. 6, 359–366 (2002).
Stockwell, B.R., Haggarty, S.J. & Schreiber, S.L. High-throughput screening of small molecules in miniaturized mammalian cell-based assays involving post-translational modifications. Chem. Biol. 6, 71–83 (1999).
Shogren-Knaak, M., Alaimo, P.J. & Shokat, K.M. Recent advances in chemical approaches to the study of biological systems. Annu. Rev. Cell Dev. Biol. 17, 405–433 (2001).
Kuruvilla, F.G., Shamji, A.F., Sternson, S.M., Hergenrother, P.J. & Schreiber, S.L. Dissecting glucose signalling with diversity-oriented synthesis and small-molecule microarrays. Nature 416, 653–657 (2002).
Guo, Z., Zhou, D. & Schultz, P.G. Designing small-molecule switches for protein-protein interactions. Science 288, 2042–2045 (2000).
Tawa, P., Tam, J., Cassady, R., Nicholson, D.W. & Xanthoudakis, S. Quantitative analysis of fluorescent caspase substrate cleavage in intact cells and identification of novel inhibitors of apoptosis. Cell Death Differ. 8, 30–37 (2001).
Fowler, A. et al. An evaluation of fluorescence polarization and lifetime discriminated polarization for high throughput screening of serine/threonine kinases. Anal. Biochem. 308, 223–231 (2002).
Parker, G.J., Law, T.L., Lenoch, F.J. & Bolger, R.E. Development of high throughput screening assays using fluorescence polarization: nuclear receptor-ligand-binding and kinase/phosphatase assays. J. Biomol. Screen. 5, 77–88 (2000).
Levine, L.M., Michener, M.L., Toth, M.V. & Holwerda, B.C. Measurement of specific protease activity utilizing fluorescence polarization. Anal. Biochem. 247, 83–88 (1997).
Salisbury, C.M., Maly, D.J. & Ellman, J.A. Peptide microarrays for the determination of protease substrate specificity. J. Am. Chem. Soc. 124, 14868–14870 (2002).
Dixon, T.C., Meselson, M., Guillemin, J. & Hanna, P.C. Anthrax. N. Engl. J. Med. 341, 815–826 (1999).
Inglesby, T.V. et al. Anthrax as a biological weapon: medical and public health management. Working group on civilian biodefense. JAMA 281, 1735–1745 (1999).
Petosa, C., Collier, R.J., Klimpel, K.R., Leppla, S.H. & Liddington, R.C. Crystal structure of the anthrax toxin protective antigen. Nature 385, 833–838 (1997).
Bradley, K.A., Mogridge, J., Mourez, M., Collier, R.J. & Young, J.A. Identification of the cellular receptor for anthrax toxin. Nature 414, 225–229 (2001).
Varughese, M., Teixeira, A.V., Liu, S. & Leppla, S.H. Identification of a receptor-binding region within domain 4 of the protective antigen component of anthrax toxin. Infect. Immun. 67, 1860–1865 (1999).
Drum, C.L. et al. Structural basis for the activation of anthrax adenylyl cyclase exotoxin by calmodulin. Nature 415, 396–402 (2002).
Pellizzari, R., Guidi-Rontani, C., Vitale, G., Mock, M. & Montecucco, C. Lethal factor of Bacillus anthracis cleaves the N-terminus of MAPKKs: analysis of the intracellular consequences in macrophages. Int. J. Med. Microbiol. 290, 421–427 (2000).
Duesbery, N.S. et al. Proteolytic inactivation of MAP-kinase-kinase by anthrax lethal factor. Science 280, 734–737 (1998).
Vitale, G., Bernardi, L., Napolitani, G., Mock, M. & Montecucco, C. Susceptibility of mitogen-activated protein kinase kinase family members to proteolysis by anthrax lethal factor. Biochem. J. 352, 739–745 (2000).
Pannifer, A.D. et al. Crystal structure of the anthrax lethal factor. Nature 414, 229–233 (2001).
Friedlander, A.M. Tackling anthrax. Nature 414, 160–161 (2001).
Tonello, F., Seveso, M., Marin, O., Mock, M. & Montecucco, C. Screening inhibitors of anthrax lethal factor. Nature 418, 386 (2002).
Houseman, B.T., Huh, J.H., Kron, S.J. & Mrksich, M. Peptide chips for the quantitative evaluation of protein kinase activity. Nat. Biotechnol. 20, 270–274 (2002).
Houseman, B.T., Gawalt, E.S. & Mrksich, M. Maleimide-functionalized self-assembled monolayers for the preparation of peptide and carbohydrate biochips. Langmuir 19, 1522–1531 (2003).
Cummings, R.T. et al. A peptide-based fluorescence resonance energy transfer assay for Bacillus anthracis lethal factor protease. Proc. Natl. Acad. Sci. USA 99, 6603–6606 (2002).
Sigal, G.B., Mrksich, M. & Whitesides, G.M. Effect of surface wettability on the adsorption of proteins and detergents. J. Am. Chem. Soc. 120, 3464–3473 (1998).
Mrksich, M. & Whitesides, G.M. Using self-assembled monolayers that present oligo(ethylene glycol) groups to control the interactions of proteins with surfaces. Am. Chem. Soc. Symp. Ser. 680, 361–373 (1997).
Trevor, J.L., Lykke, K.R., Pellin, M.J. & Hanley, L. Two-laser mass spectrometry of thiolate, disulfide, and sulfide self-assembled monolayers. Langmuir 14, 1664–1673 (1998).
Su, J. & Mrksich, M. Using mass spectrometry to characterize self-assembled monolayers presenting peptides, proteins, and carbohydrates. Angew. Chem. Int. Ed. Engl. 41, 4715–4718 (2002).
Koo, H.M. et al. Apoptosis and melanogenesis in human melanoma cells induced by anthrax lethal factor inactivation of mitogen-activated protein kinase kinase. Proc. Natl. Acad. Sci. USA 99, 3052–3057 (2002).
Mosmann, T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 65, 55–63 (1983).
Zhang, J.H., Chung, T.D. & Oldenburg, K.R. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J. Biomol. Screen. 4, 67–73 (1999).
Houseman, B.T. & Mrksich, M. Carbohydrate arrays for the evaluation of protein binding and enzymatic modification. Chem. Biol. 9, 443–454 (2002).
Moy, F.J. et al. MS/NMR: a structure-based approach for discovering protein ligands and for drug design by coupling size exclusion chromatography, mass spectrometry, and nuclear magnetic resonance spectroscopy. Anal. Chem. 73, 571–581 (2001).
Acknowledgements
This work was supported by the National Science Foundation, the Ludwig Fund for Cancer Research and a Burroughs Wellcome Graduate Fellowship to D.-H.M.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Min, DH., Tang, WJ. & Mrksich, M. Chemical screening by mass spectrometry to identify inhibitors of anthrax lethal factor. Nat Biotechnol 22, 717–723 (2004). https://doi.org/10.1038/nbt973
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt973
This article is cited by
-
Exploration of the nanomedicine-design space with high-throughput screening and machine learning
Nature Biomedical Engineering (2019)
-
Effects of metalloprotease anthrax lethal factor on its peptide-based inhibitor R9LF-1
Molecular and Cellular Biochemistry (2015)
-
Analysis of alkanethiolates on gold with matrix-assisted laser desorption/ionization time-of-flight mass spectrometry
Journal of the Korean Society for Applied Biological Chemistry (2015)
-
Three-component reaction discovery enabled by mass spectrometry of self-assembled monolayers
Nature Chemistry (2012)
-
Spatial localization of bacteria controls coagulation of human blood by 'quorum acting'
Nature Chemical Biology (2008)