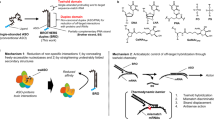
Abstract
Transcription factors are important targets for the treatment of a variety of malignancies but are extremely difficult to inhibit, as they are located in the cell's nucleus and act mainly by protein-DNA and protein-protein interactions. The transcriptional regulators Id1 and Id3 are attractive targets for cancer therapy as they are required for tumor invasiveness, metastasis and angiogenesis. We report here the development of an antitumor agent that downregulates Id1 effectively in tumor endothelial cells in vivo. Efficient delivery and substantial reduction of Id1 protein levels in the tumor endothelium were effected by fusing an antisense molecule to a peptide known to home specifically to tumor neovessels. In two different tumor models, systemic delivery of this drug led to enhanced hemorrhage, hypoxia and inhibition of primary tumor growth and metastasis, similar to what is observed in Id1 knockout mice. Combination with the Hsp90 inhibitor 17-(allylamino)-17-demethoxygeldanamycin yielded virtually complete growth suppression of aggressive breast tumors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Emanuel, B.S. et al. The 2p breakpoint of a 2;8 translocation in Burkitt lymphoma interrupts the V kappa locus. Proc. Natl. Acad. Sci. USA 81, 2444–2446 (1984).
Mushinski, J.F., Potter, M., Bauer, S.R. & Reddy, E.P. DNA rearrangement and altered RNA expression of the c-myb oncogene in mouse plasmacytoid lymphosarcomas. Science 220, 795–798 (1983).
Vogelstein, B. & Kinzler, K.W. Cancer genes and the pathways they control. Nat. Med. 10, 789–799 (2004).
Darnell, J.E., Jr. Transcription factors as targets for cancer therapy. Nat. Rev. Cancer 2, 740–749 (2002).
Wang, L.H., Yang, X.Y., Kirken, R.A., Resau, J.H. & Farrar, W.L. Targeted disruption of stat6 DNA binding activity by an oligonucleotide decoy blocks IL-4-driven T(H)2 cell response. Blood 95, 1249–1257 (2000).
Turkson, J. et al. Novel peptidomimetic inhibitors of signal transducer and activator of transcription 3 dimerization and biological activity. Mol. Cancer Ther. 3, 261–269 (2004).
Jing, N. et al. G-quartet oligonucleotides: a new class of signal transducer and activator of transcription 3 inhibitors that suppresses growth of prostate and breast tumors through induction of apoptosis. Cancer Res. 64, 6603–6609 (2004).
Mateo-Lozano, S. et al. Combined transcriptional and translational targeting of EWS/FLI-1 in Ewing's sarcoma. Clin. Cancer Res. 12, 6781–6790 (2006).
Sumida, T., Itahana, Y., Hamakawa, H. & Desprez, P.Y. Reduction of human metastatic breast cancer cell aggressiveness on introduction of either form a or B of the progesterone receptor and then treatment with progestins. Cancer Res. 64, 7886–7892 (2004).
Lee, K.T. et al. Overexpression of Id-1 is significantly associated with tumour angiogenesis in human pancreas cancers. Br. J. Cancer 90, 1198–1203 (2004).
Vandeputte, D.A. et al. Expression and distribution of id helix-loop-helix proteins in human astrocytic tumors. Glia 38, 329–338 (2002).
Minn, A.J. et al. Genes that mediate breast cancer metastasis to lung. Nature 436, 518–524 (2005).
Gupta, G.P. et al. ID genes mediate tumor re-initiation during breast cancer lung metastasis. Proc. Natl. Acad. Sci. USA (in the press).
Lyden, D. et al. Id1 and Id3 are required for neurogenesis, angiogenesis and vascularization of tumour xenografts. Nature 401, 670–677 (1999).
de Candia, P. et al. Angiogenesis impairment in Id-deficient mice cooperates with an Hsp90 inhibitor to completely suppress HER2/neu-dependent breast tumors. Proc. Natl. Acad. Sci. USA 100, 12337–12342 (2003).
Ruzinova, M.B. et al. Effect of angiogenesis inhibition by Id loss and the contribution of bone-marrow-derived endothelial cells in spontaneous murine tumors. Cancer Cell 4, 277–289 (2003).
Li, H., Gerald, W.L. & Benezra, R. Utilization of bone marrow-derived endothelial cell precursors in spontaneous prostate tumors varies with tumor grade. Cancer Res. 64, 6137–6143 (2004).
Perk, J. et al. Reassessment of id1 protein expression in human mammary, prostate, and bladder cancers using a monospecific rabbit monoclonal anti-id1 antibody. Cancer Res. 66, 10870–10877 (2006).
Sakurai, D. et al. Crucial role of inhibitor of DNA binding/differentiation in the vascular endothelial growth factor-induced activation and angiogenic processes of human endothelial cells. J. Immunol. 173, 5801–5809 (2004).
Belletti, B. et al. Regulation of Id1 protein expression in mouse embryo fibroblasts by the type 1 insulin-like growth factor receptor. Exp. Cell Res. 277, 107–118 (2002).
Borlak, J., Meier, T., Halter, R., Spanel, R. & Spanel-Borowski, K. Epidermal growth factor-induced hepatocellular carcinoma: gene expression profiles in precursor lesions, early stage and solitary tumours. Oncogene 24, 1809–1819 (2005).
Viloria-Petit, A. et al. Acquired resistance to the antitumor effect of epidermal growth factor receptor-blocking antibodies in vivo: a role for altered tumor angiogenesis. Cancer Res. 61, 5090–5101 (2001).
Casanovas, O., Hicklin, D.J., Bergers, G. & Hanahan, D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell 8, 299–309 (2005).
Porkka, K., Laakkonen, P., Hoffman, J.A., Bernasconi, M. & Ruoslahti, E. A fragment of the HMGN2 protein homes to the nuclei of tumor cells and tumor endothelial cells in vivo. Proc. Natl. Acad. Sci. USA 99, 7444–7449 (2002).
Lyden, D. et al. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat. Med. 7, 1194–1201 (2001).
Ohtani, N. et al. Opposing effects of Ets and Id proteins on p16(INK4a) expression during cellular senescence. Nature 409, 1067–1070 (2001).
Fong, S. et al. Id-1 as a molecular target in therapy for breast cancer cell invasion and metastasis. Proc. Natl. Acad. Sci. USA 100, 13543–13548 (2003).
Christian, S. et al. Nucleolin expressed at the cell surface is a marker of endothelial cells in angiogenic blood vessels. J. Cell Biol. 163, 871–878 (2003).
Shi, H. et al. Nucleolin is a receptor that mediates antiangiogenic and anti-tumor activity of endostatin. Blood 110, 2899–2906 (2007).
Zhao, Q. et al. Cellular distribution of phosphorothioate oligonucleotide following intravenous administration in mice. Antisense Nucleic Acid Drug Dev. 8, 451–458 (1998).
Solit, D.B. et al. 17-Allylamino-17-demethoxygeldanamycin induces the degradation of androgen receptor and HER-2/neu and inhibits the growth of prostate cancer xenografts. Clin. Cancer Res. 8, 986–993 (2002).
Holmgren, L., O'Reilly, M.S. & Folkman, J. Dormancy of micrometastases: balanced proliferation and apoptosis in the presence of angiogenesis suppression. Nat. Med. 1, 149–153 (1995).
Doubrovin, M., Serganova, I., Mayer-Kuckuk, P., Ponomarev, V. & Blasberg, R.G. Multimodality in vivo molecular-genetic imaging. Bioconjug. Chem. 15, 1376–1388 (2004).
Ponomarev, V. et al. A novel triple-modality reporter gene for whole-body fluorescent, bioluminescent, and nuclear noninvasive imaging. Eur. J. Nucl. Med. Mol. Imaging 31, 740–751 (2004).
Perk, J., Iavarone, A. & Benezra, R. Id family of helix-loop-helix proteins in cancer. Nat. Rev. Cancer 5, 603–614 (2005).
Ruzinova, M.B. & Benezra, R. Id proteins in development, cell cycle and cancer. Trends Cell Biol. 13, 410–418 (2003).
Akerman, M.E., Chan, W.C., Laakkonen, P., Bhatia, S.N. & Ruoslahti, E. Nanocrystal targeting in vivo. Proc. Natl. Acad. Sci. USA 99, 12617–12621 (2002).
Reddy, G.R. et al. Vascular targeted nanoparticles for imaging and treatment of brain tumors. Clin. Cancer Res. 12, 6677–6686 (2006).
Corey, D.R. 48000-fold Acceleration of hybridization by chemically modified oligonucleotides. J. Am. Chem. Soc. 117, 9373–9374 (1995).
Winkler, F. et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell 6, 553–563 (2004).
Shaked, Y. et al. Therapy-induced acute recruitment of circulating endothelial progenitor cells to tumors. Science 313, 1785–1787 (2006).
Mabjeesh, N.J. et al. Geldanamycin induces degradation of hypoxia-inducible factor 1alpha protein via the proteosome pathway in prostate cancer cells. Cancer Res. 62, 2478–2482 (2002).
Petit, A.M. et al. Neutralizing antibodies against epidermal growth factor and ErbB-2/neu receptor tyrosine kinases down-regulate vascular endothelial growth factor production by tumor cells in vitro and in vivo: angiogenic implications for signal transduction therapy of solid tumors. Am. J. Pathol. 151, 1523–1530 (1997).
Henry, S.P., Grillone, L.R., Orr, J.L., Bruner, R.H. & Kornbrust, D.J. Comparison of the toxicity profiles of ISIS 1082 and ISIS 2105, phosphorothioate oligonucleotides, following subacute intradermal administration in Sprague-Dawley rats. Toxicology 116, 77–88 (1997).
Trepel, M., Arap, W. & Pasqualini, R. Modulation of the immune response by systemic targeting of antigens to lymph nodes. Cancer Res. 61, 8110–8112 (2001).
Arap, M.A. et al. Cell surface expression of the stress response chaperone GRP78 enables tumor targeting by circulating ligands. Cancer Cell 6, 275–284 (2004).
Kolonin, M.G., Saha, P.K., Chan, L., Pasqualini, R. & Arap, W. Reversal of obesity by targeted ablation of adipose tissue. Nat. Med. 10, 625–632 (2004).
Ardelt, P.U. et al. Targeting urothelium: ex vivo assay standardization and selection of internalizing ligands. J. Urol. 169, 1535–1540 (2003).
Lee, L. et al. Identification of synovium-specific homing peptides by in vivo phage display selection. Arthritis Rheum. 46, 2109–2120 (2002).
Nowakowski, G.S. et al. A specific heptapeptide from a phage display peptide library homes to bone marrow and binds to primitive hematopoietic stem cells. Stem Cells 22, 1030–1038 (2004).
Harrison, J.G. & Balasubramanian, S. Synthesis and hybridization analysis of a small library of peptide-oligonucleotide conjugates. Nucleic Acids Res. 26, 3136–3145 (1998).
Acknowledgements
The authors thank Simona Curelariu for help with animal models and Ninche Alston for help with in vivo imaging. This work was supported by the Deutsche Forschungsgemeinschaft (fellowship to E.H.), the National Institutes of Health (R.B.), William H. Goodwin and Alice Goodwin and the Commonwealth Cancer Foundation for Research and the Experimental Therapeutics Center of Memorial Sloan-Kettering Cancer Center (R.B.), the Breast Cancer Research Foundation (R.B.) and the Mary Kay Ash Foundation (R.B.).
Author information
Authors and Affiliations
Contributions
All authors contributed significantly to the experimental design and/or execution of the experiments described.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–12 (PDF 968 kb)
Rights and permissions
About this article
Cite this article
Henke, E., Perk, J., Vider, J. et al. Peptide-conjugated antisense oligonucleotides for targeted inhibition of a transcriptional regulator in vivo. Nat Biotechnol 26, 91–100 (2008). https://doi.org/10.1038/nbt1366
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt1366
This article is cited by
-
Image-based modeling of vascular organization to evaluate anti-angiogenic therapy
Biology Direct (2023)
-
Id proteins: emerging roles in CNS disease and targets for modifying neural stemcell behavior
Cell and Tissue Research (2022)
-
Generation of complex human organoid models including vascular networks by incorporation of mesodermal progenitor cells
Scientific Reports (2019)
-
The Id-protein family in developmental and cancer-associated pathways
Cell Communication and Signaling (2017)
-
ID Proteins Regulate Diverse Aspects of Cancer Progression and Provide Novel Therapeutic Opportunities
Molecular Therapy (2014)