Abstract
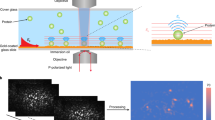
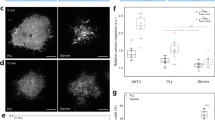
G protein–coupled receptors (GPCRs) constitute an abundant family of membrane receptors of high pharmacological interest. Cell-based assays are the predominant means of assessing GPCR activation, but are limited by their inherent complexity. Functional molecular assays that directly and specifically report G protein activation by receptors could offer substantial advantages. We present an approach to immobilize receptors stably and with defined orientation to substrates. By surface plasmon resonance (SPR), we were able to follow ligand binding, G protein activation, and receptor deactivation of a representative GPCR, bovine rhodopsin. Microcontact printing was used to produce micrometer-sized patterns with high contrast in receptor activity. These patterns can be used for local referencing to enhance the sensitivity of chip-based assays. The immobilized receptor was stable both for hours and during several activation cycles. A ligand dose–response curve with the photoactivatable agonist 11-cis-retinal showed a half-maximal signal at 120 nM. Our findings may be useful to develop novel assay formats for GPCRs based on receptor immobilization to solid supports, particularly to sensor surfaces.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Gudermann, T., Kalkbrenner, F. & Schultz, G. Diversity and selectivity of receptor-G protein interaction. Annu. Rev. Pharmacol. Toxicol. 36, 429– 459 (1996).
Stadel, J.M., Wilson, S. & Bergsma, D.J. Orphan G protein-coupled receptors: a neglected opportunity for pioneer drug discovery. Trends Pharmacol. Sci. 18, 430–437 (1997).
Helmreich, E.J.M. & Hofmann, K.-P. Structure and function of proteins in G protein-coupled signal transfer. Biochim. Biophys. Acta. 1286, 285–322 (1996).
Fivash, M., Towler, E.M. & Fisher, R.J. BIAcore for macromolecular interaction. Curr. Opin. Biotechnol. 9, 97–101 (1998).
Hermanson, G.T., Mallia, A.K. & Smith, P.K. Immobilized affinity ligand techniques. (Academic Press, San Diego, CA; 1992).
Bruckert, F., Vuong, T.M. & Chabre, M. Light and GTP dependence of transducin solubility in retinal rods. Eur. Biophys. J. 16, 207– 218 (1988).
Heyse, S., Ernst, O.P., Dienes, Z., Hofmann, K.-P. & Vogel, H. Incorporation of rhodopsin in laterally structured supported membranes: observation of transducin activation with spatially and time-resolved surface plasmon resonance. Biochemistry 37, 507–522 (1998).
Vanrhee, A.M. & Jacobson, K.A. Molecular architecture of G-Protein-coupled receptors. Drug Dev. Res. 37, 1– 38 (1996).
O'Shannessy, D.J. & Quarles, R.H. Specific conjugation reactions of the oligosaccharide moieties of immunoglobulins. J. Appl. Biochem. 7, 347–355 (1985).
Bain, C.D. & Whitesides, G.M. Modeling organic surfaces with self-assembled monolayers. Angew. Chem. Int. Ed. 101 , 522–528 (1989).
Spinke, J. et al. Molecular recognition at self-assembled monolayers - optimization of surface functionalization. J. Chem. Phys. 99, 7012 –7019 (1993).
Xia, Y. & Whitesides, G.M. Soft lithography. Angew. Chem. Int. Ed. 37, 550–575 (1998).
Sackmann, E. Supported membranes: scientific and practical applications. Science 271, 43–48 (1996).
Lang, H., Duschl, C. & Vogel, H. A new class of thiolipids for the attachment of lipid bilayers on gold surfaces. Langmuir 10, 197–210 (1994).
Farrens, D.L. & Khorana, H.G. Structure and function in rhodopsin. Measurement of the rate of metarhodopsin II decay by fluorescence spectroscopy. J. Biol. Chem. 270, 5073– 5076 (1995).
Antonny, B., Otto-Bruc, A., Chabre, M. & Vuong, T.M. GTP hydrolysis by purified alpha-subunit of transducin and its complex with the cyclic GMP phosphodiesterase inhibitor. Biochemistry 32, 8646–8653 (1993).
Freissmuth, M., Waldhoer, M., Bofill-Cardona, E. & Nanoff, C. G protein antagonists. Trends Pharmacol. Sci. 20, 237–245 (1999).
Hamm, H.E. et al. Site of G protein binding to rhodopsin mapped with synthetic peptides from the alpha subunit. Science 241, 832– 835 (1988).
Hovius, R. et al. Fluorescence techniques for fundamental and applied studies of membrane protein receptors: the 5-HT3 serotonin receptor. J. Recept. Signal Transduct. Res. 19, 533–545 (1999).
Remmers, A.E., Posner, R. & Neubig, R.R. Fluorescent guanine nucleotide analogs and G protein activation. J. Biol. Chem. 269, 13771– 13778 (1994).
Papermaster, D.S. Preparation of antibodies to rhodopsin and the large protein of rod outer segments. Methods Enzymol. 81, 240– 246 (1982).
Kühn, H. Interaction of the rod cell proteins with the disk membrane: influence of light, ionic strength and nucleotides. Curr. Top. Membr. Transp. 15, 171–201 (1981).
Heck, M. & Hofmann, K.P. G-protein-effector coupling: a real-time light-scattering assay for transducin-phosphodiesterase interaction. Biochemistry 32, 8220– 8227 (1993).
Molday, R.S. & MacKenzie, D. Monoclonal antibodies to rhodopsin: characterization, cross-reactivity, and application as structural probes. Biochemistry 22, 653–660 (1983).
Delamarche, E. et al. Transport mechanisms of alkanethiols during microcontact printing on gold. J. Phys. Chem. B 102, 3324– 3334 (1998).
Arnis, S. & Hofmann, K.P. Photoregeneration of bovine rhodopsin from its signaling state. Biochemistry 34, 9333–9340 (1995).
Acknowledgements
We thank Dr. A. Brecht, D. Stamou, and Dr. R. Hovius for advice and critical reading of the manuscript; A. Heusler for the synthesis of the alkane thiols; and Dr. E. Delamarche (IBM, Zürich, Switzerland) for offering microcontact printing stamps. This research was supported by the Board of the Swiss Federal Institutes of Technology (SPP MINAST, 7.06) to H.V., the Deutsche Forschungsgemeinschaft (SFB 449) to K.P.H. and O.P.E., and the Fonds der Chemischen Industrie to K.P.H.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bieri, C., Ernst, O., Heyse, S. et al. Micropatterned immobilization of a G protein–coupled receptor and direct detection of G protein activation. Nat Biotechnol 17, 1105–1108 (1999). https://doi.org/10.1038/15090
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/15090