Abstract
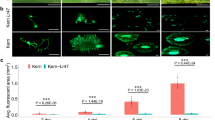
Selectable markers of bacterial origin such as the neomycin phosphotransferase type II gene, which can confer kanamycin resistance to transgenic plants, represent an invaluable tool for plant engineering. However, since all currently used antibiotic-resistance genes are of bacterial origin, there have been concerns about horizontal gene transfer from transgenic plants back to bacteria, which may result in antibiotic resistance. Here we characterize a plant gene, Atwbc19, the gene that encodes an Arabidopsis thaliana ATP binding cassette (ABC) transporter and confers antibiotic resistance to transgenic plants. The mechanism of resistance is novel, and the levels of resistance achieved are comparable to those attained through expression of bacterial antibiotic-resistance genes in transgenic tobacco using the CaMV 35S promoter. Because ABC transporters are endogenous to plants, the use of Atwbc19 as a selectable marker in transgenic plants may provide a practical alternative to current bacterial marker genes in terms of the risk for horizontal transfer of resistance genes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Higgins, C.F. ABC transporters—from microorganisms to man. Annu. Rev. Cell Biol. 8, 67–113 (1992).
Sanchez-Fernandez, R., Rea, P.A., Davies, T.G. & Coleman, J.O. Do plants have more genes than humans? Yes, when it comes to ABC proteins. Trends Plant Sci. 6, 347–348 (2001).
Sanchez-Fernandez, R., Davies, T.G.E., Coleman, J.O.D. & Rea, P.A. The Arabidopsis thaliana ABC protein superfamily, a complete inventory. J. Biol. Chem. 276, 30231–30244 (2001).
Pighin, J.A. et al. Plant cuticular lipid export requires an ABC transporter. Science 306, 702–704 (2004).
Mingeot-Leclercq, M.P., Glupczynski, Y. & Tulkens, P.M. Aminoglycosides: activity and resistance. Antimicrob. Agents Chemother. 43, 727–737 (1999).
Vasquez, D. Translation inhibitors. Int. Rev. Biochem. Biophys 30, 1–306 (1979).
Bar-Nun, S., Shneyour, Y. & Beckman, J.S. G418, an elongation inhibitor of 80S ribosomes. Biochim. Biophys. Acta 741, 123–127 (1983).
Wright, G.D., Berghuis, A.M. & Mobashery, S. Aminoglycoside antibiotics—structures, functions, and resistance. Adv. Exp. Med. Biol. 456, 27–69 (1998).
Bevan, M.W., Flavell, R.B. & Chilton, M.D. A chimaeric antibiotic-resistance gene as a selectable marker for plant-cell transformation. Nature 304, 184–187 (1983).
Fraley, R.T. et al. Expression of bacterial genes in plant cells. Proc. Natl. Acad. Sci. USA 80, 4803–4807 (1983).
Herrera-Estrella, L. et al. Chimeric genes as dominant selectable markers in plant cells. EMBO J. 2, 987–995 (1983).
Miki, B. & McHugh, S. Selectable marker genes in transgenic plants: applications, alternatives and biosafety. J. Biotechnol. 107, 193–232 (2004).
Kage, K. et al. Dominant-negative inhibition of breast cancer resistance protein as drug efflux pump through the inhibition of S-S dependent homodimerization. Int. J. Cancer 97, 626–630 (2002).
Dreesen, T.D., Johnson, D.H. & Henikoff, S. The brown protein of Drosophila melanogaster is similar to the white protein and to components of active transport complexes. Mol. Cell. Biol. 12, 5206–5215 (1988).
Heinemann, J.A. & Traavik, T. Problems in monitoring horizontal gene transfer in field trials of transgenic plants. Nat. Biotechnol. 22, 1105–1109 (2004).
Nielsen, K.M. & Townsend, J.P. Monitoring and modeling horizontal gene transfer. Nat. Biotechnol. 22, 1110–1114 (2004).
Davison, J. Monitoring horizontal gene transfer. Nat. Biotechnol. 22, 1349 (2004).
Day, A. Antibiotic resistance genes in transgenic plants: their origins, undesirability and technologies for their elimination from genetically modified crops. in Transgenic Plants: Current Innovations and Future Trends (ed. Stewart C.N. Jr.) 111–156, (Horizon Scientific Press, Norfolk, England, 2003).
Rommens, C.M. et al. Crop improvement through modification of the plant's own genome. Plant Physiol. 135, 421–431 (2004).
Rommens, C.M. All-native DNA transformation: a new approach to plant genetic engineering. Trends Plant Sci. 9, 457–464 (2004).
Goddijn, O.J., van der Duyn Schouten, P.M., Schilperoort, R.A. & Hoge, J.H. A chimaeric tryptophan decarboxylase gene as a novel selectable marker in plant cells. Plant Mol. Biol. 22, 907–912 (1993).
Zuo, J., Niu, Q.W., Ikeda, Y. & Chua, N.H. Marker-free transformation: increasing transformation frequency by the use of regeneration- promoting genes. Curr. Opin. Biotechnol. 13, 173–180 (2002).
Liu, H.K., Yang, C. & Wei, Z.M. Efficient Agrobacterium tumefaciens-mediated transformation of soybeans using an embryonic tip regeneration system. Planta 219, 1042–1049 (2004).
Zhang, B.H., Liu, F., Liu, Z.H., Wang, H.M. & Yao, C.B. Effects of kanamycin on tissue culture and somatic embryogenesis in cotton. Plant Growth Regul. 33, 137–149 (2001).
Poulsen, G.B. Genetic transformation of Brassica. Plant Breed. 115, 209–225 (1996).
Agharbaoui, Z., Greer, A.-F. & Tabaeizadeh, Z. Transformation of the wild tomato Lycopersicon chilense Dun. by Agrobacterium tumefaciens. Plant Cell Rep. 15, 102–105 (1995).
van Frankenhuyzen, K. & Beardmore, T. Current status and environmental impact of transgenic forest trees. Can. J. For. Res. 34, 1163–1180 (2004).
Horsch, R.B. et al. A simple and general-method for transferring genes into plants. Science 227, 1229–1231 (1985).
Jefferson, R.A., Kavanagh, T.A. & Bevan, M.W. GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 6, 3901–3907 (1987).
Harper, B.K. et al. Green fluorescent protein in transgenic plants indicates the presence and expression of a second gene. Nat. Biotechnol. 17, 1125–1129 (1999).
Acknowledgements
We are grateful to John Dunlap for his assistance in confocal microscopy imaging and Andreas Nebenführ for helpful discussions as well as improvement suggested by three anonymous reviewers. We wish to thank Megan York, Jason Burris, Jaime Davis and Reginald Millwood for assistance in lab and greenhouse work. This research work was supported by The University of Tennessee Institute of Agriculture and the US Army.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Root growth and NPTII synthesis levels in control Arabidopsis thaliana Col1 and homozygous insertional knockout mutants (KO). (PDF 854 kb)
Supplementary Fig. 2
Twelve-week old non-transgenic and T1 ABC-transgenic tobacco plants in the greenhouse. (PDF 124 kb)
Supplementary Fig. 3
Root length distribution frequency of non-transgenic tobacco (Xanthi), and T1 transgenic lines 28 and 30 grown on MSO media with or without 100 or 200 mg/l kanamycin. (PDF 160 kb)
Supplementary Fig. 4
T-DNA region of construct used for subcellular localization of AtWBC19. (PDF 603 kb)
Supplementary Fig. 5
Clustal alignment of the Arabidopsis thaliana WBC members most closely related to Atwbc19. (PDF 394 kb)
Supplementary Table 1
Phenotypic data of 9-week-old greenhouse-grown T1 tobacco plants. (PDF 37 kb)
Supplementary Table 2
Transformation of canola (Brassica napus) cv. Westar hypocotyl segments with plasmids pNPT and pABC. (PDF 40 kb)
Rights and permissions
About this article
Cite this article
Mentewab, A., Stewart, C. Overexpression of an Arabidopsis thaliana ABC transporter confers kanamycin resistance to transgenic plants. Nat Biotechnol 23, 1177–1180 (2005). https://doi.org/10.1038/nbt1134
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt1134
This article is cited by
-
Identification and expression analysis of ATP-binding cassette (ABC) transporters revealed its role in regulating stress response in pear (Pyrus bretchneideri)
BMC Genomics (2024)
-
Mutations in BrABCG26, encoding an ATP-binding cassette transporter, are responsible for male sterility in Chinese cabbage (Brassica rapa L. ssp. pekinensis)
Theoretical and Applied Genetics (2024)
-
What is the impact of aminoglycoside exposure on soil and plant root-associated microbiota? A systematic review protocol
Environmental Evidence (2022)
-
Genome-wide analysis of ATP-binding cassette transporter provides insight to genes related to bioactive metabolite transportation in Salvia miltiorrhiza
BMC Genomics (2021)
-
Agrobacterium tumefaciens mediated transformation of the aquatic carnivorous plant Utricularia gibba
Plant Methods (2020)