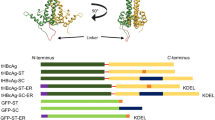
Abstract
Here we present data showing oral immunogenicity of recombinant hepatitis B surface antigen (HBsAg) in preclinical animal trials. Mice fed transgenic HBsAg potato tubers showed a primary immune response (increases in HBsAg-specific serum antibody) that could be greatly boosted by intraperitoneal delivery of a single subimmunogenic dose of commercial HBsAg vaccine, indicating that plants expressing HBsAg in edible tissues may be a new means for oral hepatitis B immunization. However, attainment of such a goal will require higher HBsAg expression than was observed for the potatoes used in this study. We conducted a systematic analysis of factors influencing the accumulation of HBsAg in transgenic potato, including 5′ and 3′ flanking elements and protein targeting within plant cells. The most striking improvements resulted from (1) alternative polyadenylation signals, and (2) fusion proteins containing targeting signals designed to enhance integration or retention of HBsAg in the endoplasmic reticulum (ER) of plant cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Muraskin, W. The war against hepatitis B. A history of the International Task Force on Hepatitis B Immunization. (University of Pennsylvania Press, Philadelphia, PA; 1995).
Bruss, V., Gerhardt, E., Vieluf, K. & Wunderlich, G. Functions of the large hepatitis B virus surface protein in viral particle morphogenesis. Intervirology 39, 23– 31 (1996).
Wamplar, D., Lehman, E., Boger, J., McAleer, W. & Scolnick, E. Multiple chemical forms of the hepatitis B surface antigen produced in yeast. Proc. Natl. Acad. Sci. USA 82, 6830–6834 ( 1985).
Murray, L. et al. Hepatitis B virus antigens made in microbial cells immunise against viral infection. EMBO J. 3, 645– 650 (1984).
Szmuness W. et al. Hepatitis B vaccine: demonstration of efficacy in a controlled clinical trial in a high-risk population in the United States. N. Engl. J. Med. 303, 833–841 (1980).
Anonymous. Centers for Disease Control. Hepatitis B virus: a comprehensive strategy for eliminating transmission in the United States through universal childhood vaccination. Recommendations of the Immunization Practices Advisory Committee (ACIP). Morbidity Mortality Weekly Rep 40, 1–25 ( 1991).
Eble, B., MacRae, D., Lingappa, V. & Ganem, D. Multiple topogenic sequences determine the transmembrane orientation of hepatitis B surface antigen. Mol. Cell. Biol. 7, 3591 –3601 (1987).
Prevelige, P.J. Inhibiting virus–capsid assembly by altering the polymerisation pathway . Trends Biotechnol. 16, 61– 65 (1998).
World Health Organization. The World Health Report 1998 Executive summary: life in the 21st century–a vision for all. (1998).
Mason, H.S., Lam, D.M.K. & Arntzen, C.J. Expression of hepatitis B surface antigen in transgenic plants. Proc. Natl. Acad. Sci. USA 89, 11745 –11749 (1992).
Thanavala, Y., Yang, Y.-F., Lyons, P., Mason, H.S. & Arntzen, C.J. Immunogenicity of transgenic plant-derived hepatitis B surface antigen. Proc. Natl. Acad. Sci. USA 92, 3358–3361 (1995).
Aizpurua, H.J.D. & Russell-Jones, G.J. Oral vaccination: identification of classes of proteins that provoke an immune response upon oral feeding. J. Exp. Med. 167, 440 (1988).
Wenzler, H., Mignery, G., Fisher, L. & Park, W. Analysis of a chimeric class-I patatin-GUS gene in transgenic potato plants: high-level expression in tubers and sucrose-inducible expression in cultured leaf and stem explants . Plant Mol. Biol. 12, 41– 50 (1989).
Florence, A.T. & Jani, P.U. Particulate delivery: the challenge of the oral route. In Pharmaceutical particulate carriers: therapeutic applications (ed. Rolland, A.) 65– 107 (Marcel Dekker, Inc., New York, NY; 1993).
Dogan B.M.H., Richter, L., Hunter, J.B. & Shuler, M.L. Process options in hepatitis B surface antigen extraction from transgenic potato. Biotechnol. Progr. 16, 435– 441(2000).
Suh, S.G., Stiekma, W.J. & Hannapel, D.J. Proteinase inhibitor activity and wound-inducible gene expression of the 22-kDa potato tuber proteins. Planta (Heidelberg) 184, 423–430 ( 1991).
Chan, M. & Yu, S. The 3′ untranslated region of a rice alpha-amylase gene functions as a sugar-dependent mRNA stability determinant. Proc. Natl. Acad. Sci. USA 95, 6543–6547 (1998).
Mason, H., Guerrero, F., Boyer, J. & Mullet, J. Proteins homologous to leaf storage proteins are abundant in stems of soybean seedlings. Analysis of proteins and cDNAs. Plant Mol. Biol. 11, 845–856 (1988).
DeWald, D. (PhD Thesis) Enzymatic activity, localization, and gene expression of the soybean vegetative storage proteins, VSPα and VSPβ. (Texas A&M University) (1992).
Gomord, V. et al. The C-terminal HDEL sequence is sufficient for retention of secretory proteins in the endoplasmic reticulum (ER) but promotes vacuolar targeting of proteins that escape the ER. Plant J. 11, 313–325 (1997).
Wandelt, C.I. et al. Vicilin with carboxy-terminal KDEL is retained in the endoplasmic reticulum and accumulates to high levels in the leaves of transgenic plants . Plant J. 2, 181–192 (1992).
Huovila A.-P.J., Eder, A.M. & Fuller, S.D. Hepatitis B surface antigen assembles in a post-ER, pre-Golgi compartment. J. Cell Biol. 118, 1305–1320 (1992).
Neuhaus, J. & Rogers, J. Sorting of proteins to vacuoles in plant cells. Plant Mol. Biol. 38, 127–144 (1998).
Bednarek, S. & Raikhel, N. Intracellular trafficking of secretory proteins. Plant Mol. Biol. 20, 133–150 (1992).
Nawrath, C., Poirier, Y. & Sommerville, C. Targeting of the hydroxybutyrate biosynthetic pathway to the plastids of Arabidopsis thaliana results in high levels of polymer accumulation. Proc. Natl. Acad. Sci. USA 91, 12760–12764 (1994).
Haq, T.A., Mason, H.S., Clements, J.D. & Arntzen, C.J. Oral immunization with a recombinant bacterial antigen produced in transgenic plants. Science 268, 714– 716 (1995).
Jefferson, R., Kavanagh, T. & Bevan, M. GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 13, 3901–3907 ( 1987).
Becker, D., Kemper, E., Schell, J. & Masterson, R. New plant binary vectors with selectable markers located proximal to the left T-DNA border . Plant Mol. Biol. 20, 1195– 1197 (1992).
An, G. et al. Functional analysis of the 3′ control region of the potato wound-inducible proteinase inhibitor II gene. Plant Cell 1, 115–122 (1989).
Mason, H., Haq, T., Clements, J. & Arntzen, C. Edible vaccine protects mice against E. coli heat-labile enterotoxin (LT): potatoes expressing a synthetic LT-B gene. Vaccine 16, 1336–1343 (1998).
Acknowledgements
The authors wish to thank Qingxian Kong for performing the oral immunization experiments. This work was supported by a grant from the National Institutes of Health No. AI42836 to Y.T., C.J.A., and H.S.M.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Richter, L., Thanavala, Y., Arntzen, C. et al. Production of hepatitis B surface antigen in transgenic plants for oral immunization. Nat Biotechnol 18, 1167–1171 (2000). https://doi.org/10.1038/81153
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/81153
This article is cited by
-
Phyto-factories of anti-cancer compounds: a tissue culture perspective
Beni-Suef University Journal of Basic and Applied Sciences (2022)
-
Edible Vaccines: Promises and Challenges
Molecular Biotechnology (2020)
-
Virus-based pharmaceutical production in plants: an opportunity to reduce health problems in Africa
Virology Journal (2019)
-
An intronless form of the tobacco extensin gene terminator strongly enhances transient gene expression in plant leaves
Plant Molecular Biology (2018)