Abstract
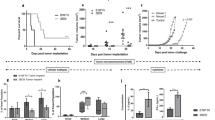
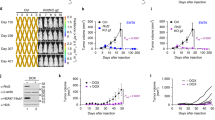
We describe a simple technology used to cure an established metastatic disease. Intradermal injection of plasmid DNA encoding a transcriptionally targeted cytotoxic gene, along with hsp70, not only promoted tissue-specific, inflammatory killing of normal melanocytes, but also induced a CD8+ T-cell–dependent, antigen-specific response in mice that eradicated systemically established B16 tumors. This CD8+ T cell response was subsequently suppressed in vivo within a few days. The data demonstrate that deliberate destruction of normal tissue can be exploited to generate immunity against a malignant disease originating from that tissue. This approach obviates the need to identify tumor antigens and does not require complex isolation of tumor cells or their derivatives. In addition, it provides a model system for studying the mechanisms underlying the etiology and control of autoimmune diseases. Finally, despite targeting normal tissue, therapy could be separated from development of overt autoimmune symptoms, suggesting that the strategy may be valuable against tumors derived from both non-essential and essential tissue types.
*Note: In the version of this article originally published online, the name of one of the authors was spelled incorrectly. Mayoi Lai should be Maoyi Lai. This mistake has been corrected in the HTML version and will appear correctly in print.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others

Change history
15 August 2004
corrected name; inserted footnote into abs and full text; appended corrigendum pdf to AOP PDF; corrected online date will appear in print
References
Dudley, M.E. et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science 298, 850–854 (2002).
Pardoll, D.M. Spinning molecular immunology into successful immunotherapy. Nat. Rev. Immunol. 2, 227–238 (2002).
Hodi, F.S. et al. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc. Natl. Acad. Sci. USA 100, 4712–4717 (2003).
Phan, G.Q. et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc. Natl. Acad. Sci. USA 100, 8372–8377 (2003).
Srivastava, P.K. Hypothesis: controlled necrosis as a tool for immunotherapy of human cancer. Cancer Immun. 3, 4 (2003).
Huang, A.Y.C. et al. Role of bone marrow derived cells in presenting MHC Class I-restricted tumor antigens. Science 264, 961–965 (1994).
Gallucci, S., Lolkema, M. & Matzinger, P. Natural adjuvants: endogenous activators of dendritic cells. Nat. Med. 5, 1249–1255 (1999).
Melcher, A.A., Gough, M.J., Todryk, S. & Vile, R.G. Apoptosis or necrosis for tumour immunotherapy—what's in a name? J. Mol. Med. 77, 824–833 (1999).
Liu, K. et al. Immune tolerance after delivery of dying cells to dendritic cells in situ. J. Exp. Med. 196, 1091–1097 (2002).
Mougneau, E., Hugues, S. & Glaichenhaus, N. Antigen presentation by dendritic cells in vivo. J. Exp. Med. 196, 1013–1016 (2002).
Melcher, A.A. et al. Tumor immunogenicity is determined by the mechanism of cell death via induction of heat shock protein expression. Nat. Med. 4, 581–587 (1998).
Todryk, S. et al. Heat shock protein 70 induced during tumor cell killing induces Th1 cytokines and targets immature dendritic cell precursors to enhance antigen uptake. J. Immunol. 163, 1398–1408 (1999).
Gough, M.J. et al. Macrophages orchestrate the immune response to tumor cell death. Cancer Res. 61, 7240–7247 (2001).
Restifo, N.P. Building better vaccines: how apoptotic cell death can induce inflammation and activate innate and adaptive immunity. Curr. Opin. Immunol. 12, 597–603 (2000).
MacAry, P.A. et al. HSP70 peptide binding mutants separate antigen delivery from dendritic cell stimulation. Immunity 20, 95–106 (2004).
Asea, A. et al. Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J. Biol. Chem. 277, 15028–15034 (2002).
Engelhard, V.H., Bullock, T.N., Colella, T.A., Sheasley, S.L. & Mullins, D.W. Antigens derived from melanocyte differentiation proteins: self-tolerance, autoimmunity, and use for cancer immunotherapy. Immunol. Rev. 188, 136–146 (2002).
Bronte, V. et al. Genetic vaccination with “self” tyrosinase-related protein 2 causes melanoma eradication but not vitiligo. Cancer Res. 60, 253–258 (2000).
Parmiani, G. Tumor immunity as autoimmunity: tumor antigens include normal self proteins which stimulate anergic peripheral T cells. Immunol. Today 14, 536–538 (1993).
Pardoll, D.M. Inducing autoimmune disease to treat cancer. Proc. Natl. Acad. Sci. USA 96, 5340–5342 (1999).
van Elsas, A. et al. Elucidating the autoimmune and antitumor effector mechanisms of a treatment based on cytotoxic T lymphocyte antigen-4 blockade in combination with a B16 melanoma vaccine: comparison of prophylaxis and therapy. J. Exp. Med. 194, 481–489 (2001).
Paus, R. & Cotsarelis, G. The biology of hair follicles. N. Engl. J. Med. 341, 491–497 (1999).
Alexeev, V. & Yoon, K. Stable and inheritable changes in genotype and phenotype of albino melanocytes induced by an RNA-DNA oligonucleotide. Nat. Biotechnol. 16, 1343–1346 (1998).
Alexeev, V. & Yoon, K. Gene correction by RNA-DNA oligonucleotides. Pigment Cell Res. 13, 72–79 (2000).
Alexeev, V., Igoucheva, O., Domashenko, A., Cotsarelis, G. & Yoon, K. Localized in vivo genotypic and phenotypic correction of the albino mutation in skin by RNA-DNA oligonucleotide. Nat. Biotechnol. 18, 43–47 (2000).
Saito, N. et al. High efficiency genetic modification of hair follicles and growing hair shafts. Proc. Natl. Acad. Sci. USA 99, 13120–13124 (2002).
Vile, R.G. & Hart, I.R. Use of tissue-specific expression of the Herpes Simplex Virus thymidine kinase gene to inhibit growth of established murine melanomas following direct intratumoral injection of DNA. Cancer Res. 53, 3860–3864 (1993).
Vile, R.G. & Hart, I.R. In vitro and in vivo targeting of gene expression to melanoma cells. Cancer Res. 53, 962–967 (1993).
Diaz, R.M., Eisen, T., Hart, I.R. & Vile, R.G. Exchange of viral promoter/enhancer elements with heterologous regulatory sequences generates targeted hybrid long terminal repeat vectors for gene therapy of melanoma. J. Virol. 72, 789–795 (1998).
Christoph, T. et al. The human hair follicle immune system: cellular composition and immune privilege. Br. J. Dermatol. 142, 862–873 (2000).
Musette, P. et al. Immune-mediated destruction of melanocytes in Halo Nevi is associated with the local expansion of a limited number of T cell clones. J. Immunol. 162, 1789–1794 (1999).
Millar, D.G. et al. Hsp70 promotes antigen-presenting cell function and converts T-cell tolerance to autoimmunity in vivo. Nat. Med. 9, 1469–1476 (2003).
Bonnotte, B. et al. Intradermal injection, as opposed to subcutaneous injection, enhances immunogenicity and suppresses tumorigenicity of tumor cells. Cancer Res. 63, 2145–2149 (2003).
Walker, L.S.K. & Abbas, A.K. The enemy within: keeping self-reactive T cells at bay in the periphery. Nat. Rev. Immunol. 2, 11–19 (2002).
Kemp, E.H., Waterman, E.A. & Weetman, A.P. Immunological pathomechanisms in vitiligo. Exp. Rev. Mol. Med. (July 23, 2001), pp. 1–22.
Konstantopoulos, K. Value of tyrosinase RNA detection by an RT-PCR method in melanoma prognosis [comment]. Br. J. Cancer 88, 981 (2003); [author reply Br. J. Cancer 88, 982 (2003)].
Valverde, P., Garcia-Borron, J.C., Martinez-Liarte, J.H., Solano, F. & Lozano, J.A. Melanocyte stimulating hormone activation of tyrosinase in B16 mouse melanoma cells. Fed. Eur. Biochem. Soc. 304, 114–118 (1992).
Kursar, M. et al. Regulatory CD4+CD25+ T cells restrict memory CD8+ T cell responses. J. Exp. Med. 196, 1585–1592 (2002).
Dyall, R. et al. Heteroclitic immunization induces tumor immunity. J. Exp. Med. 188, 1553–1561 (1998).
Hogquist, K.A. et al. T cell receptor antagonistic peptides induce positive selection. Cell 76, 17 (1994).
Hara, I. et al. Rejection of mouse melanoma elicited by local secretion of interleukin-2: implicating macrophages without T cells or natural killer cells in tumor rejection. Int. J. Cancer 61, 253–260 (1995).
Onizuka, S. et al. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor α) monoclonal antibody. Cancer Res. 59, 3128–3133 (1999).
Linardakis, E. et al. Enhancing the efficacy of a weak allogeneic melanoma vaccine by viral fusogenic membrane glycoprotein-mediated tumor cell–tumor cell fusion. Cancer Res. 62, 5495–5504 (2002).
Coligan, J.E., Kruisbeek, A.M., Margulies, D.H., Shevach, E.M. & Strober, W. Isolation of mouse mononuclear cells. In Current Protocols in Immunology, vol 1, 3.1.2–3.1.5 (John Wiley and Sons, Inc., New York, 1998).
Overwijk, W. et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J. Exp. Med. 198, 569–580 (2003).
Altman, D.G. Analysis of survival times. In Practical Statistics for Medical Research, 365–395 (Chapman & Hall/CRC, Press, London, 1991).
Ganss, R., Montoliu, L., Monaghan, A.P. & Schutz, G. A cell-specific enhancer far upstream of the mouse tyrosinase gene confers high level and copy number–related expression in transgenic mice. EMBO J. 13, 3083–3093 (1994).
Acknowledgements
This work was supported by the Mayo Foundation and by National Institutes of Health grants RO1 CA94180 and P50CA91956. We thank T. Higgins for expert secretarial assistance. The antibodies specific to CD4 and CD8 were a gift from P. Wettstein and M. Rodriguez (Mayo Clinic). The tyrosinase promoter/enhancer element was a gift of D. Schadendorf, Skin Cancer Unit, German Cancer Research Center Heidelberg & University Hospital Mannheim, Germany. Pmel transgenic T cells specific for the hgp100 epitope were a gift of N. Restifo.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Daniels, G., Sanchez-Perez, L., Diaz, R. et al. A simple method to cure established tumors by inflammatory killing of normal cells. Nat Biotechnol 22, 1125–1132 (2004). https://doi.org/10.1038/nbt1007
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt1007
This article is cited by
-
Intense immunostaining of heat shock protein 70 within renal interstitium associates with long-term renal survival in an ANCA-associated vasculitis cohort
Cell Stress and Chaperones (2021)
-
Tetanus toxoid and CCL3 improve dendritic cell vaccines in mice and glioblastoma patients
Nature (2015)
-
Immunogenic HSV-mediated Oncolysis Shapes the Antitumor Immune Response and Contributes to Therapeutic Efficacy
Molecular Therapy (2014)
-
Functional Cloning of Recurrence-specific Antigens Identifies Molecular Targets to Treat Tumor Relapse
Molecular Therapy (2013)
-
Heat shock protein 27 (HSP27): biomarker of disease and therapeutic target
Fibrogenesis & Tissue Repair (2012)