Abstract
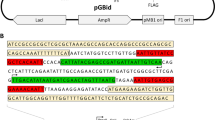
We have used the GroE chaperonins to assist in the packing of a new phage display vector, pEXmide3. Titers of the packed phagemid increased almost 200-fold from ∼ 4×1011 cfu/ml, without coexpression of the GroE proteins, to ∼7×l013 cfu/ml with their coexpression. Equal titers of non-assisted and assisted phagestocks exhibited the same antigen specificity and ELISA reactivity, indicating the same frequency of displayed Fab-fragments. While the diversity of antibody libraries depends on the bacterial transformation efficiency, the copy number of each antibody is determined by subsequent amplification of the phage, thus chaperonin assisted phagemid packing in bacteriophage M13 can be used as a general and simple tool to increase the amplification level of expressed Fab fragments. pEXmide3 was developed for display of Fab and single chain Fv-fragments (scFv), using restriction enzymes that do not cut, or cut with low frequencies, in genes encoding immunoglobulin variable domains. The vector allows cloning of genes for the variable domains Unking these to predetermined human constant domains or cloning of the entire light and heavy Fab chains. A modification of the pelB leader sequence, with a glutamine to alanine substitution at residue 18, was used for export of the light chain.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Parmley, S.F. and Smith, G.P. 1988. Antibody-selectable filamentous fd phage vectors: affinity purification of target genes. Gene 73: 305–318.
McCafferty, J., Griffiths, A.D., Winter, G. and Chiswell, F.J. 1990. Phage antibodies: Filamentous phage displaying antibody variable domains. Nature 348: 552–554.
Barbas, C.F. III, Rang, A.S., Lerner, R.A. and Benkovic, S.J. 1991. Assembly of combinatorial libraries on phage surfaces: The gene III site. Proc. Natl. Acad. Sci. USA 88: 7978–7982.
Chang, C.N., Landolfi, N.F. and Queen, C. 1991. Expression of antibody Fab domains on bacteriophage surfaces. Potential use for antibody selection. J. Immunol. 147: 3610–3614.
Garraid, L.J., Yang, M., O'Conell, M.P., Kelley, R.F. and Henner, D.J. 1991. Fab assembly and enrichment in a monovalent phage display system. Bio/Technology 9: 1373–1377.
Hoogenboom, H.R., Griffiths, A.D., Johnson, K.S., Hudson, P. and Winter, G. 1991. Multi-subunit proteins on the surface of filamentous phage: methodologies for displaying antibody (Fab) heavy and light chains. Nucl. Acids Res. 19: 4133–4137.
Kang, A.S., Barbas, C.F. III, Janda, K.D., Benkovic, S.J. and Lerner, R.A. 1991. Linkage of recognition and replication functions by assembling combinatorial antibody Fab libraries along phage surfaces. Proc. Natl. Acad. Sci. USA 88: 4363–4366.
Huse, W.D., Sastry, L., Iverson, S.A., Kang, A.S., Alting-Mees, M., Burton, D.R., Benkovic, S.J. and Lerner, R.A. 1989. Generation of a large combinatorial library of immunoglobulin repertoire in phage lambda. Science 246: 1275–1281.
Clackson, T., Hoogenboom, H.R., Griffiths, A.D. and Winter, G. 1991. Making antibody fragments using phage display libraries. Nature 352: 624–628.
Barbas, C.F., Bjorling, E., Chiodi, F., Björling, E., Cababa, D., Jones, T.M., Zebedee, S.L., Persson, M.A.A., Nar, P.L., Norrby, E. and Burton, D.R. 1992. Recombinant human Fab fragments neutralize human type 1 immunodeficiency virus in vitro . J. Mol. Biol. 89: 9339–9343.
Zebedee, S.L., Barbas, C.E. III, Horn, Y.-L., Caothien, R.H., Graff, R., DeGraw, J., Pyati, J., LaPolla, R., Burton, D.R., Lemer, R.A. and Thornton, G.B. 1992. Human combinatorial antibody libraries to hepatitis B surface antigen. Proc. Natl. Acad. Sci. USA 89: 3175–3179.
Barbas, C.F., Crowe, J.E. Jr., Cababa, D., Jones, T. M., Zebedee, S.L., Mulphy, B.R., Chanock, R.M. and Burton, D.R. 1992. Human monoclonal Fab fragments derived from a combinatorial library bind to respiratory syncytial virus F glycoprotein and neutralize infectivity. J. Mol. Biol. 89: 10164–10168.
Marks, J.D., Hoogenboom, H.R., Bonnert, T.P., McCafferty, J., Griffiths, A.D. and Whiter, G. 1991. By-passing immunization. Human antibodies from V-gene libraries displayed on phage. J. Mol. Biol. 222: 581–597.
Gram, H., Marconi, L.-A., Barbas, C.E. III, Collect, T.A., Lerner, R.A. and Kang, A.S. 1992. In vitro selection and affinity maturation of antibodies from naive combinatorial immunoglobulin library. Proc. Natl. Acad. USA 89: 3576–3580.
Hawkings, R.E., Russell, S.J. and Winter, G. 1992. Selection of phage antibodies by binding affinity. Mimicking affinity maturation. J. Mol. Biol. 226: 889–896.
Larrick, J.W., Danielsson, L., Brenner, A.C., Abrahamsson, M., Fry, K. and Borrebaeck, C.A.K. 1989. Rapid cloning of rearranged immunoglobulin genes from human hybridoma cells using mixed primers and polymerase chain reaction. Biochem. Biophys. Res. Commun. 160: 1250–1256.
Campbell, M.J., Zelenetz, A.D., Levy, S. and Levy, R. 1992. Use of family specific leader region primers for PCR amplification of the human heavy chain variable region gene repertoire. Molec. Immunol. 29: 193–203.
Danielsson, L. and Borrebaeck, C.A.K. 1992. Amplification of rearranged Ig variable region DNA from single cells, p. 89–137. In: Antibody Engineering. A Practical Guide. Borrebaeck, C. A. K. (Ed.). W. H. Freeman and Company, New York.
Chaudary, V.K., Batra, J.K., Gallo, M.G., Willingham, M.C., Fitzgerald, D.J. and Pastan, I. 1990. A rapid method of cloning functional variable antibody genes in Escherichia coli as single-chain immunotoxins. Proc. Natl. Acad. Sci. USA 87: 1066–1070.
Zeilstra-Ryalls, J., Fayet, O. and Georgopoulos, C. 1991. The universally conserved GroE (Hsp60) chaperonins. Ann. Rev. Microbiol. 45: 301–325.
Heijne, G., 1986. A new method for predicting signal sequence cleavage site. Nucl. Acids Res. 14: 4683–4690.
Ward, E.S., G, D., Griffiths, A.D., Jones, P.T. and Winter, G. 1989. Binding activities of a repertoire of immunoglobulins secreted from Escherichia coli . Nature 341: 544–549.
Borrebaeck, C.A.K., Malmborg, A.-C., Furebring, C., Michaelsson, A., Ward, S., Danielsson, L. and Ohlin, M. 1992. Kinetic analysis of recombinant antibody-antigen interactions:relation between structural domains and antigen binding. Bio/Technology 6: 697–698.
Goloubinoff, P., Gatenby, A.A. and Lorimer, G.H. 1989. GroE heat-shock proteins promote assembly of foreign prokaryotic ribulose bisphosphate carbox-ylase oligomers in Escherichia coli . Nature 337: 44–47.
Mäkelä, O., Kaartinen, M., Pelkonen, J.L.T. and Karjalainen, K. 1978. Inheritance of antibody specificity V. Anti-2-phenyloxazolone in the mouse. J. Exp. Med. 148: 1644–1660.
Ward, E.S. 1992. Expression and purification of antibody fragments using Escherichia coli as a host, p. 121–137. In: Antibody Engineering. A Practical Guide. C. A. K. Borrebaeck (Ed.). W. H. Freeman and Company, New York.
Ohlin, M. and Borrebaeck, C.A.K. 1993. Production of human monoclonal antibodies. In: Methods of Immunological Analyses, Vol. II Masseyeff, R. E, Albert, W. H. W. and Staines, N. A. (Eds.). VCH Verlagsgesellschaft mbH, Weinheim. In press.
Malmborg, A.-C., Michaelsson, A., Ohlin, M., Jansson, B. and Borrebaeck, C.A.K. 1992. Real time analysis of antibody-antigen reaction kinetics. Scand. J. Immunol. 35: 643–650.
Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166: 557–580.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Söderfind, E., Lagerkvist, A., Dueñas, M. et al. Chaperonin Assisted Phage Display of Antibody Fragments on Filamentous Bacteriophages. Nat Biotechnol 11, 503–507 (1993). https://doi.org/10.1038/nbt0493-503
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nbt0493-503