Abstract
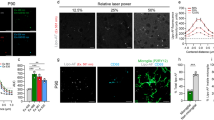
To develop a rapid method of quantifying immunohistochemical information in tissue sections, we tested a confocal laser fluorescence microscanner initially designed for DNA microarray analysis. This instrument collects digital images at multiple wavelengths, scans entire sections at a resolution of 5 or 10 μm in less than 10 min, and quantifies structures labeled with fluorescent or nonfluorescent probes. We assessed the microscanner by studying immunostained amyloid plaques in the Alzheimer's disease (AD) brain and in the brain of a transgenic mouse model of AD amyloidosis, as efforts to correlate measures of amyloid plaques in brain sections with behavioral impairments are impeded by limitations in current morphometric methods. Microscanner analysis was used to determine amyloid burden in the occipital and entorhinal cortices of the mouse (3.7%) and human AD brain (1.6%). We also quantified the colocalization of plaque b-amyloid (Ab) with glial fibrillary acidic protein, a marker of gliosis (mouse 0.9%, human AD 3.7%). The microscanner may be generally applicable to a wide variety of human histopathologies and their animal models, wherever rapid unbiased quantitative analysis is needed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Goedert, M., Trojanowski, J., and Lee, V.-Y. 1997. The neurofibrillary pathology of Alzheimer's disease, pp. 613–627, in The Molecular and genetic basis of neurological disease, 2nd ed. Prusiner, S., Rosenberg, R., Di Mauro, S., and Barchi, R. (eds.). Butterworth Heineman Press, Boston, MA.
Morrison-Bogorad, M., Weiner, M., Rosenberg, R., Bigio, E., and White, C.I. 1997. Alzheimer's disease, pp. 581–600, in The molecular and genetic basis of neurological disease, 2nd ed. Prusiner, S., Rosenberg, R., Di Mauro, S., and Barchi, R. (eds.). Butterworth Heineman Press, Boston, MA.
Selkoe, D. 1997. Cellular and molecular biology of the beta-amyloid precursor protein and Alzheimer's disease, pp. 601–612, in The molecular and genetic basis of neurological disease, 2nd ed. Prusiner, S., Rosenberg, R., Di Mauro, S., and Barchi, R. Butterworth Heineman Press, Boston, MA.
National Institute on Aging and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer's Disease. Phelps, C., Khachaturian, Z., and Trojanowski, J.Q., Working Group Chairs. 1997. Consensus recommendations for the postmortem diagnosis of Alzheimer's disease. Neurobiol Aging 18: S1– 2.
McKee, A.C., Kosik, K.S., and Kowall, N.W. 1991. Neuritic pathology and dementia in Alzheimer's disease. Ann. Neurol. 30: 156–165.
Arriagada, P.V., Growdon, J.H., Hedley-Whyte, E.T., and Hyman, B.T. 1992. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer's disease. Neurology 42: 631–639.
Dickson, D.W., Crystal, H.A., Mattiace, L.A., Masur, D.M., Blau, A.D., Davies, P. et al. 1992. Identification of normal and pathological aging in prospectively studied nondemented elderly humans. Neurobiol. Aging 13: 179–189.
Cummings, B.J., Pike, C.J., Shankle, R., and Cotman, C.W. 1996 . Beta-amyloid deposition and other measures of neuropathology predict cognitive status in Alzheimer's disease. Neurobiol. Aging 17: 921–933.
Guillery, R.W. and Herrup, K. 1997. Quantification without pontification: choosing a method for counting objects in sectioned tissues. J. Comp. Neurol. 386: 2– 7.
Games, D., Adams, D., Alessandrini, R., Barbour, R., Berthelette, P., Blackwell, C. et al. 1995. Alzheimer-type neuropathology in transgenic mice overexpressing V717F beta-amyloid precursor protein. Nature 373: 523–527.
Irizarry, M.C., Soriano, F., McNamara, M., Page, K., Schenk, D., Games, D., and Hyman, B. 1997. A-beta deposition is associated with neuropil changes, but not with overt neuronal loss in the human amyloid precursor protein V717F (PDAPP) transgenic mouse. J. Neurosci. 17: 7053– 70539.
Smith, D.H., Nakamura, M., McIntosh, T.K., Wang, J., Rodriguez, A., Chen, X. et al. 1998. Brain trauma induces massive hippocampal neuron death linked to a surge in Aβ1-42 levels in mice overexpressing mutant APP. Am. J. Pathol. 153: 1005– 1010.
Schmidt, M.L., DiDario, A.G., Lee, V.M., and Trojanowski, J.Q. 1994. An extensive network of PHF tau-rich dystrophic neurites permeates neocortex and nearly all neuritic and diffuse amyloid plaques in Alzheimer disease. FEBS Lett. 344: 69– 73.
Schmidt, M.L., Lee, V.M., Saido, T., Perl, D., Schuck, T., Iwatsubo, T., and Trojanowski, J.Q. 1998. Amyloid plaques in Guam amyotrophic lateral sclerosis/parkinsonism-dementia complex contain species of A beta similar to those found in the amyloid plaques of Alzheimer's disease and pathological aging. Acta. Neuropathol. (Berl) 95: 117–122.
Alexay, C., Kain, R., Hanzel, D., and Johnston, R. 1996 . Fluorescence scanner employing a macro scanning objective. Proc. SPIE 2705: 63-72. US patent 5646411, 5672880, 5719391.
Belichenko, P., Fedorov, A., and Dahlstrom, A. 1996. Quantitative analysis of immunofluorescence and lipofuscin distribution in human cortical areas by dual-channel confocal laser scanning microscopy. J. Neurosci. Methods 69: 155–161.
Masliah, E., Sisk, A., Mallory, M., Mucke, L., Schenk, D., and Games, D. 1996. Comparison of neurodegenerative pathology in transgenic mice overexpressing V717F beta-amyloid precursor protein and Alzheimer's disease. J. Neurosci. 16: 5795–5811.
Russ, J. 1995.The image processing handbook. CRC Press, Boca Raton, FL.
Acknowledgements
We thank V.M.-Y. Lee, D.L. Barker, Y. Nakagawa, K.B. Bechtol, and M.L. Schmidt as well as T.-H. Chiu, M.T. Schuck, and B. Kain for advice and assistance with these studies. Appreciation also is expressed to J. Clemens and members of his laboratory at Eli Lilly for making the PDAPP mice available to us for this study, and to the families of the patients studied here who made this research possible through their generosity and support. Supported by grants from the National Institute on Aging of the National Institutes of Health to J.Q.T., and by an ATP/NIST grant (70NANB5H1031) to Molecular Dynamics.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hanzel, D., Trojanowski, J., Johnston, R. et al. High-throughput quantitative histological analysis of Alzheimer's disease pathology using a confocal digital microscanner. Nat Biotechnol 17, 53–57 (1999). https://doi.org/10.1038/5225
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/5225
This article is cited by
-
The role of astrocytic α7 nicotinic acetylcholine receptors in Alzheimer disease
Nature Reviews Neurology (2023)
-
A VNS based framework for early diagnosis of the Alzheimer's disease converted from mild cognitive impairment
Optimization Letters (2023)
-
Association of glial and neuronal degeneration markers with Alzheimer’s disease cerebrospinal fluid profile and cognitive functions
Alzheimer's Research & Therapy (2020)
-
Resveratrol Protects Optic Nerve Head Astrocytes from Oxidative Stress-Induced Cell Death by Preventing Caspase-3 Activation, Tau Dephosphorylation at Ser422 and Formation of Misfolded Protein Aggregates
Cellular and Molecular Neurobiology (2020)
-
Generation of transgenic cynomolgus monkeys that express green fluorescent protein throughout the whole body
Scientific Reports (2016)