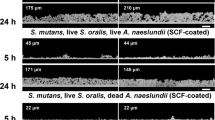
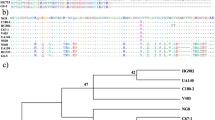
Abstract
The earliest step in microbial infection is adherence by specific microbial adhesins to the mucosa of the oro-intestinal, nasorespiratory, or genitourinary tract. We inhibited binding of a cell surface adhesin of Streptococcus mutans to salivary receptors in vitro, as measured by surface plasmon resonance, using a synthetic peptide (p1025) corresponding to residues 1025–1044 of the adhesin. Two residues within p1025 that contribute to binding (Q1025, E1037) were identified by site-directed mutagenesis. In an in vivo human streptococcal adhesion model, direct application of p1025 to the teeth prevented recolonization of S. mutans but not Actinomyces , as compared with a control peptide or saline. This novel antimicrobial strategy, applying competitive peptide inhibitors of adhesion, may be used against other microorganisms in which adhesins mediate colonization of mucosal surfaces.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Loesche, W.J., Rowan, J., Straffon, L.H., and Loos, P.J. 1975. Association of Streptococcus mutans with human dental decay. Infect. Immun. 11: 1252– 1260.
Russell, M.W. and Lehner, T. 1978. Characterisation of antigens extracted from cells and culture fluids of Streptococcus mutans serotype c. Arch. Oral Biol. 23: 7– 15.
Russell, R.R.B. 1979. Wall–associated protein antigens of Streptococcus mutans. J. Gen. Microbiol. 114: 109–115.
Lehner, T., Russell, M.W., and Caldwell, J. 1980. Immunisation with a purified protein from Streptococcus mutans against dental caries in rhesus monkeys. Lancet. i: 995–996.
Lehner, T., Challacombe, S.J., and Caldwell, J. 1975. Immunological and bacteriological basis for vaccination against dental caries in rhesus monkeys. Nature 254: 517–520.
Ericson, T. and Rundegren, J. 1983. Characterization of a salivary agglutinin reacting with a serotype c strain of Streptococcus mutans. Eur. J. Biochem. 133: 255– 261.
Russell, M.W., and Mansson–Rahemtulla, B. 1989 . Interaction between surface protein antigens of Streptococcus mutans and human salivary components. Oral Microbiol. Immunol . 4: 106–111.
Crowley, P.J., Brady, L.J., Piacentini, D.A., and Bleiweis, A.S. 1993. Identification of a salivary agglutinin–binding domain within cell surface adhesin P1 of Streptococcus mutans. Infect. Immun. 61: 1547–1552.
Munro, G.H., Evans, P., Todryk, S., Buckett, P., Kelly, C.G., and Lehner, T. 1993. A protein fragment of streptococcal cell surface antigen I/II which prevents adhesion of Streptococcus mutans. Infect. Immun. 61: 4590–4598.
Kelly, C.G., Todryk, S., Kendal, H.L., Munro, G.H., and Lehner, T. 1995. T cell, adhesion and B cell epitopes of the cell surface Streptococcus mutans protein antigen I/II. Infect. Immun. 63: 3649– 3638.
Ma, J–K.C., Hunjan, M., Smith, R., and Lehner, T. 1989. Specificity of monoclonal antibodies in local passive immunization against Streptococcus mutans. Clin. Exper. Immunol. 77: 331–337.
Boman, H.G. 1995. Peptide antibiotics and their role in innate immunity. Annu. Rev. Immunol. 13: 61– 92.
Homonylo–McGavin, M.K., and Lee, S.F. 1996. Role of the C terminus in antigen P1 surface localization in Streptococcus mutans and two related cocci. J. Bacteriol . 178: 801–807.
Lehner, T., Caldwell, J., and Smith, R. 1987. Local passive immunization by monoclonal antibodies against streptococcal antigen I/II in the prevention of dental caries. Infect. Immun. 50: 1274 –1278.
Wüthrich, K. 1986. NMR of Proteins and Nucleic Acids. John Wiley & Sons Inc, New York.
Wishart, D.S., Sykes, B.D., and Richards, F.M. 1992. The chemical shift index: a fast and simple method for the assignment of protein secondary structure through NMR spectroscopy. Biochemistry. 31: 1647–1651.
Merutka, G., Dyson, H.J., and Wright, P.E. 1995. 'Random coil' 1H chemical shifts obtained as a function of temperature and trifluoroethanol concentration for the peptide series GGXGG. J. Biomol. NMR. 5: 14–24.
Munoz, V. and Serrano, L. 1995. Elucidating the folding problem of helical peptides using empirical parameters. II. Helix macrodipole effects and rational modification of the helical content of natural peptides. J. Mol. Biol. 245: 275– 296.
Munoz, V. and Serrano, L. 1995. Elucidating the folding problem of helical peptides using empirical parameters. III. Temperature and pH dependence. J. Mol. Biol. 245: 297 –308.
Dyson, H.J., Sayre, J.R., Merutka, G., Shin, H.C., Lerner, R.A., and Wright, P.E. 1992 . Folding of peptide fragments comprising the complete sequence of proteins. Models for initiation of protein folding. II. Plastocyanin. J. Mol. Biol. 226: 819–835.
Oho, T., Yu, H., Yamashita, Y., and Koga T. 1998. Binding of salivary glycoprotein–secretory immunoglobulin A complex to the surface protein antigen of Streptococcus mutans. Infect. Immun. 66: 115–121.
Jenkinson, H.F. and Demuth, D.R. 1997 . Structure, function and immunogenicity of streptococcal antigen I/II polypeptides. Mol. Microbiol. 23: 183 –190.
Demuth, D.R., Golub, E.E., and Malamud, D. 1990. Streptococcal–host interactions. Structural and functional analysis of a streptococcus sanguis receptor for a human salivary glycoprotein. J. Biol. Chem. 265: 7120–7126.
Quiocho, F.A. 1986. Carbohydrate–binding proteins: tertiary structures and protein–sugar interactions. Annu. Rev. Biochem. 55: 287–315.
Campbell, A.C., Wong, W.Y., Houston, M. Jr., Schweizer, F., Cachia, P.J., Irvin, R.T. et al. 1997. Interaction of the receptor binding domains of Pseudomonas aeruginosa pili strains PAK, PAO, KB7 and P1 to a cross–reactive antibody and receptor analog: implications for synthetic vaccine design. J. Mol. Biol. 267: 382– 402.
Emsley, P., Charles, I.G., Fairweather, N.F., and Isaacs, N.W. 1996. Structure of Bordetella pertussis virulence factor P69 pertactin. Nature 381: 90– 92.
Zopf, D. and Roth, S. 1996. Oligosaccharide anti–infective agents. Lancet 347: 1017–1021.
Idanpaan–Heikkila, I., Simon, P.M., Zopf, D., Vullo, T., Cahill, P., Sokol, K. et al. 1997. Oligosaccharides interfere with the establishment and progression of experimental pneumococcal pneumonia. J. Infect. Dis. 176: 704–712.
Lee, K.K., Wong, W.Y., Sheth, H.B., Hodges, R.S., Paranchych, W., and Irvin, R.T. 1995. Use of synthetic peptides in characterization of microbial adhesins. Methods Enzymol. 253: 115–131.
Relman, D., Tuomanen, E., Falkow, S., Golenbock, D.T., Saukkonen, K., and Wright, S.D. 1990. Recognition of a bacterial adhesion by an integrin: macrophage CR3 (M2, CD11b/CD18) [Please Confirm] binds filamentous hemagglutinin of Bordetella pertussis. Cell 61: 1375–1382.
Putney, S.D. and Burke, P.A. 1998. Improving protein therapeutics with sustained–release formulations. Nat. Biotechnol. 16: 153–157.
Sloan–Lancaster, J. and Allen, P.M. 1996 . Altered peptide ligand–induced partial T cell activation: molecular mechanisms and role in T cell biology. Annu. Rev. Immunol. 14: 1–27.
Lee, S.F., Progulske–Fox, A., Erdos, G.W., Piacentini, D.A., Ayakawa, G.Y., Crowley, P.J. et al. 1989. Construction and characterization of isogenic mutants of Streptococcus mutans deficient in major surface protein antigen P1 (I/II). Infect. Immun. 57: 3306–3313.
Beighton, D., Russell, R.R.B., and Whiley, R.A. 1991. A simple biochemical scheme for the differentiation of Streptococcus mutans and streptococcus sobrinus . Caries Res. 25: 174–178 .
Zylber, L.J. and Jordan, H.V. 1982. Development of a selective medium for detection and enumeration of Actinomyces viscosus and Actinomyces naeslundii in dental plaque. J. Clin. Microbiol. 15: 253–259.
Davis, D.G. and Bax, A. 1985. Assignment of complex 1H–NMR spectra via two–dimensional homonuclear Hartmann–Hahn spectroscopy. J. Am. Chem. Soc. 107: 2820–2821.
Jeener, J., Meier. B.H., Bavchmann, P., and Ernst, R.R. 1979. Investigation of exchange processes by two dimensional NMR spectroscopy. J. Chem. Phys. 71: 4546– 4553.
Rance, M., Sorensen, O.W., Bodenhausen, G., Wagner, G., Ernst, R.R., and Wuthrich, K. 1983. Improved spectral resolution in cosy 1H NMR spectra of proteins via double quantum filtering. Biochem. Biophys. Res. Commun. 117: 479– 485.
Piotto, M., Saudek, V., and Sklenar, V. 1992. Gradient–tailored excitation for single–quantum NMR spectroscopy of aqueous solutions. J. Biomol. NMR. 2: 661–665.
Acknowledgements
This work was supported by the Wellcome Trust grant 040412 and the Special Funds of Guy's Hospital Dental School. NMR spectroscopic studies were supported by the TMR program "Access to Large-Scale Facilities" of the EC (ERBFMGECT950032). The BIAcore facility was established with funds from the Special Trustees of Guy's Hospital and the Special Trustees for St. Thomas' Hospital.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kelly, C., Younson, J., Hikmat, B. et al. A synthetic peptide adhesion epitope as a novel antimicrobial agent. Nat Biotechnol 17, 42–47 (1999). https://doi.org/10.1038/5213
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/5213
This article is cited by
-
Novel peptide–protein assay for identification of antimicrobial peptides by fluorescence quenching
Analytical and Bioanalytical Chemistry (2012)
-
Innate immunity glycoprotein gp-340 variants may modulate human susceptibility to dental caries
BMC Infectious Diseases (2007)
-
Peptide Inhibitors of Streptococcus mutans in the Control of Dental Caries
International Journal of Peptide Research and Therapeutics (2007)
-
Therapy of mucosal candidiasis by expression of an anti-idiotype in human commensal bacteria
Nature Biotechnology (2000)
-
Hope for the post-antibiotic era?
Nature Biotechnology (1999)