Abstract
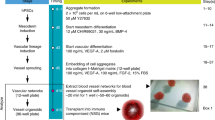
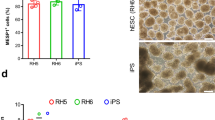
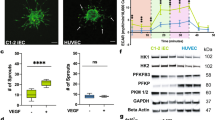
The ability to differentiate human pluripotent stem cells into endothelial cells with properties of cord-blood endothelial colony–forming cells (CB-ECFCs) may enable the derivation of clinically relevant numbers of highly proliferative blood vessel–forming cells to restore endothelial function in patients with vascular disease. We describe a protocol to convert human induced pluripotent stem cells (hiPSCs) or embryonic stem cells (hESCs) into cells similar to CB-ECFCs at an efficiency of >108 ECFCs produced from each starting pluripotent stem cell. The CB-ECFC-like cells display a stable endothelial phenotype with high clonal proliferative potential and the capacity to form human vessels in mice and to repair the ischemic mouse retina and limb, and they lack teratoma formation potential. We identify Neuropilin-1 (NRP-1)-mediated activation of KDR signaling through VEGF165 as a critical mechanism for the emergence and maintenance of CB-ECFC-like cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
Lin, Y., Weisdorf, D.J., Solovey, A. & Hebbel, R.P. Origins of circulating endothelial cells and endothelial outgrowth from blood. J. Clin. Invest. 105, 71–77 (2000).
Medina, R.J., O'Neill, C.L., Humphreys, M.W., Gardiner, T.A. & Stitt, A.W. Outgrowth endothelial cells: characterization and their potential for reversing ischemic retinopathy. Invest. Ophthalmol. Vis. Sci. 51, 5906–5913 (2010).
Moubarik, C. et al. Transplanted late outgrowth endothelial progenitor cells as cell therapy product for stroke. Stem Cell Rev. 7, 208–220 (2011).
Yoder, M.C. et al. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood 109, 1801–1809 (2007).
Ingram, D.A. et al. Vessel wall-derived endothelial cells rapidly proliferate because they contain a complete hierarchy of endothelial progenitor cells. Blood 105, 2783–2786 (2005).
Ingram, D.A. et al. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood 104, 2752–2760 (2004).
Critser, P.J., Kreger, S.T., Voytik-Harbin, S.L. & Yoder, M.C. Collagen matrix physical properties modulate endothelial colony forming cell-derived vessels in vivo. Microvasc. Res. 80, 23–30 (2010).
Schwarz, T.M. et al. Vascular incorporation of endothelial colony-forming cells is essential for functional recovery of murine ischemic tissue following cell therapy. Arterioscler. Thromb. Vasc. Biol. 32, e13–e21 (2012).
Kang, K.T., Coggins, M., Xiao, C., Rosenzweig, A. & Bischoff, J. Human vasculogenic cells form functional blood vessels and mitigate adverse remodeling after ischemia reperfusion injury in rats. Angiogenesis 16, 773–784 (2013).
Huang, X.T. et al. Intracerebroventricular transplantation of ex vivo expanded endothelial colony-forming cells restores blood-brain barrier integrity and promotes angiogenesis of mice with traumatic brain injury. J. Neurotrauma 30, 2080–2088 (2013).
Heo, S.C. et al. WKYMVm-induced activation of formyl peptide receptor 2 stimulates ischemic neovasculogenesis by promoting homing of endothelial colony forming cells. Stem Cells 32, 779–790 (2013).
Stroncek, J.D., Ren, L.C., Klitzman, B. & Reichert, W.M. Patient-derived endothelial progenitor cells improve vascular graft patency in a rodent model. Acta Biomater. 8, 201–208 (2012).
Thomson, J.A. et al. Embryonic stem cell lines derived from human blastocysts. Science 282, 1145–1147 (1998).
Yu, J. et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science 324, 797–801 (2009).
Cimato, T. et al. Neuropilin-1 identifies endothelial precursors in human and murine embryonic stem cells before CD34 expression. Circulation 119, 2170–2178 (2009).
Choi, K.D. et al. Hematopoietic and endothelial differentiation of human induced pluripotent stem cells. Stem Cells 27, 559–567 (2009).
James, D. et al. Expansion and maintenance of human embryonic stem cell-derived endothelial cells by TGFbeta inhibition is Id1 dependent. Nat. Biotechnol. 28, 161–166 (2010).
Goldman, O. et al. A boost of BMP4 accelerates the commitment of human embryonic stem cells to the endothelial lineage. Stem Cells 27, 1750–1759 (2009).
Feng, Q. et al. Hemangioblastic derivatives from human induced pluripotent stem cells exhibit limited expansion and early senescence. Stem Cells 28, 704–712 (2010).
Sone, M. et al. Pathway for differentiation of human embryonic stem cells to vascular cell components and their potential for vascular regeneration. Arterioscler. Thromb. Vasc. Biol. 27, 2127–2134 (2007).
Ginsberg, M. et al. Efficient direct reprogramming of mature amniotic cells into endothelial cells by ETS factors and TGFbeta suppression. Cell 151, 559–575 (2012).
Zachary, I.C. How neuropilin-1 regulates receptor tyrosine kinase signalling: the knowns and known unknowns. Biochem. Soc. Trans. 39, 1583–1591 (2011).
Evseenko, D. et al. Mapping the first stages of mesoderm commitment during differentiation of human embryonic stem cells. Proc. Natl. Acad. Sci. USA 107, 13742–13747 (2010).
Cho, S.W. et al. Improvement of postnatal neovascularization by human embryonic stem cell derived endothelial-like cell transplantation in a mouse model of hindlimb ischemia. Circulation 116, 2409–2419 (2007).
Kuilman, T., Michaloglou, C., Mooi, W.J. & Peeper, D.S. The essence of senescence. Genes Dev. 24, 2463–2479 (2010).
Herzog, B., Pellet-Many, C., Britton, G., Hartzoulakis, B. & Zachary, I.C. VEGF binding to NRP1 is essential for VEGF stimulation of endothelial cell migration, complex formation between NRP1 and VEGFR2, and signaling via FAK Tyr407 phosphorylation. Mol. Biol. Cell 22, 2766–2776 (2011).
Pan, Q. et al. Blocking neuropilin-1 function has an additive effect with anti-VEGF to inhibit tumor growth. Cancer Cell 11, 53–67 (2007).
Uniewicz, K.A., Cross, M.J. & Fernig, D.G. Exogenous recombinant dimeric neuropilin-1 is sufficient to drive angiogenesis. J. Biol. Chem. 286, 12–23 (2011).
Lippmann, E.S. et al. Derivation of blood-brain barrier endothelial cells from human pluripotent stem cells. Nat. Biotechnol. 30, 783–791 (2012).
Lee, M.R. et al. Epigenetic regulation of NANOG by miR-302 cluster-MBD2 completes induced pluripotent stem cell reprogramming. Stem Cells 31, 666–681 (2013).
Broxmeyer, H.E. et al. Hematopoietic stem/progenitor cells, generation of induced pluripotent stem cells, and isolation of endothelial progenitors from 21- to 23.5-year cryopreserved cord blood. Blood 117, 4773–4777 (2011).
Acknowledgements
These studies were supported by funds provided by the Riley Children's Foundation (M.C.Y.), by an AHA postdoctoral fellowship (N.P.), and Public Health Service Grants R01 HL109602 (M.C.Y. and S.L.V.-H.), RO1 HL056416 (H.E.B.), RO1 HL067384 (H.E.B.), PO1 DK090948 (H.E.B. and M.C.Y.), and by the Bio & Medical Technology Development Program of the National Research Foundation (NRF), which is funded by the South Korean government (MEST; No. 2011-0019487). The authors thank H.P. Poudel (Indiana University Purdue University Indianapolis) for critical input in biostatistical analysis. The authors also thank H.J. Lee (CHA Stem Cell Institute, CHA University, South Korea) for HLI experiments.
Author information
Authors and Affiliations
Contributions
N.P., M.R.L., H.E.B., S.R., M.P.M., A.W.S., S.L.V.-H. and M.C.Y. all participated in the design of the experiments. N.P., M.R.L., S.V., J.L.M., M.Y., M.J.F., A.F., M.G., B.M.R., M.R.S., M.G., O.E., Y.L., H.M.C., K.S.H., E.R., C.L.O. and R.J.M. performed the experiments. N.P. and M.C.Y. wrote the manuscript, and M.R.L., H.E.B., A.W.S., M.G., M.Y., A.F., C.L.O., H.M.C. and K.S.H. provided manuscript edits.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–9 and Supplementary Tables 1 and 2 (PDF 3760 kb)
Rights and permissions
About this article
Cite this article
Prasain, N., Lee, M., Vemula, S. et al. Differentiation of human pluripotent stem cells to cells similar to cord-blood endothelial colony–forming cells. Nat Biotechnol 32, 1151–1157 (2014). https://doi.org/10.1038/nbt.3048
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt.3048
This article is cited by
-
Stem cells and diabetic retinopathy: From models to treatment
Molecular Biology Reports (2023)
-
Advances in cell therapies using stem cells/progenitors as a novel approach for neurovascular repair of the diabetic retina
Stem Cell Research & Therapy (2022)
-
High-efficient serum-free differentiation of endothelial cells from human iPS cells
Stem Cell Research & Therapy (2022)
-
Endothelial and hematopoietic hPSCs differentiation via a hematoendothelial progenitor
Stem Cell Research & Therapy (2022)
-
Engineering the multiscale complexity of vascular networks
Nature Reviews Materials (2022)