Abstract
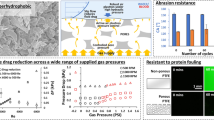
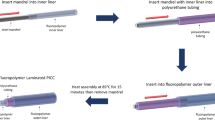
Thrombosis and biofouling of extracorporeal circuits and indwelling medical devices cause significant morbidity and mortality worldwide. We apply a bioinspired, omniphobic coating to tubing and catheters and show that it completely repels blood and suppresses biofilm formation. The coating is a covalently tethered, flexible molecular layer of perfluorocarbon, which holds a thin liquid film of medical-grade perfluorocarbon on the surface. This coating prevents fibrin attachment, reduces platelet adhesion and activation, suppresses biofilm formation and is stable under blood flow in vitro. Surface-coated medical-grade tubing and catheters, assembled into arteriovenous shunts and implanted in pigs, remain patent for at least 8 h without anticoagulation. This surface-coating technology could reduce the use of anticoagulants in patients and help to prevent thrombotic occlusion and biofouling of medical devices.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
McCarthy, P.M. & Smith, W.A. Mechanical circulatory support–a long and winding road. Science 295, 998–999 (2002).
Shen, J.I., Mitani, A.A., Chang, T.I. & Winkelmayer, W.C. Use and safety of heparin-free maintenance hemodialysis in the USA. Nephrol. Dial. Transplant. 28, 1589–1602 (2013).
von Segesser, L.K. et al. Risk and benefit of low systemic heparinization during open heart operations. Ann. Thorac. Surg. 58, 391–398 (1994).
Conn, G. et al. Is there an alternative to systemic anticoagulation, as related to interventional biomedical devices? Expert Rev. Med. Devices 3, 245–261 (2006).
Werner, C., Maitz, M.F. & Sperling, C. Current strategies towards hemocompatible coatings. J. Mater. Chem. 17, 3376–3384 (2007).
Cronin, R.E. & Reilly, R.F. Unfractionated heparin for hemodialysis: still the best option. Semin. Dial. 23, 510–515 (2010).
Finkel, K.W. & Podoll, A.S. Complications of continuous renal replacement therapy. Semin. Dial. 22, 155–159 (2009).
Shepherd, G., Mohorn, P., Yacoub, K. & May, D.W. Adverse drug reaction deaths reported in United States vital statistics, 1999–2006. Ann. Pharmacother. 46, 169–175 (2012).
Peppas, N. & Langer, R. New challenges in biomaterials. Science 263, 1715–1720 (1994).
Larm, O., Larsson, R. & Olsson, P. A new non-thrombogenic surface prepared by selective covalent binging of heparin via a modified reducing terminal residue. Biomater. Med. Devices Artif. Organs 11, 161–173 (1983).
Bannan, S. et al. Low heparinization with heparin-bonded bypass circuits: is it a safe strategy? Ann. Thorac. Surg. 63, 663–668 (1997).
Lobato, R.L. et al. Anticoagulation management during cardiopulmonary bypass: A survey of 54 North American institutions. J. Thorac. Cardiovasc. Surg. 139, 1665–1666 (2010).
Thiara, A.S. et al. Comparable biocompatibility of Phisio- and Bioline-coated cardiopulmonary bypass circuits indicated by the inflammatory response. Perfusion 25, 9–16 (2010).
Palanzo, D.A. et al. Effect of Carmeda® BioActive Surface coating versus Trillium™ Biopassive Surface coating of the oxygenator on circulating platelet count drop during cardiopulmonary bypass. Perfusion 16, 279–283 (2001).
Suhara, H. et al. Efficacy of a new coating material, PMEA, for cardiopulmonary bypass circuits in a porcine model. Ann. Thorac. Surg. 71, 1603–1608 (2001).
Smith, R.S. et al. Vascular catheters with a nonleaching poly-sulfobetaine surface modification reduce thrombus formation and microbial attachment. Sci. Transl. Med. 4, 153ra132 (2012).
Kutay, V. et al. Biocompatibility of heparin-coated cardiopulmonary bypass circuits in coronary patients with left ventricular dysfunction is superior to PMEA-coated circuits. J. Card. Surg. 21, 572–577 (2006).
Reser, D. et al. Retrospective analysis of outcome data with regards to the use of Phisio®-, Bioline®- or Softline®-coated cardiopulmonary bypass circuits in cardiac surgery. Perfusion 27, 530–534 (2012).
Bohn, H.F. & Federle, W. Insect aquaplaning: Nepenthes pitcher plants capture prey with the peristome, a fully wettable water-lubricated anisotropic surface. Proc. Natl. Acad. Sci. USA 101, 14138–14143 (2004).
Wong, T.-S. et al. Bioinspired self-repairing slippery surfaces with pressure-stable omniphobicity. Nature 477, 443–447 (2011).
Clark, L. Jr. & Gollan, F. Survival of mammals breathing organic liquids equilibrated with oxygen at atmospheric pressure. Science 152, 1755–1756 (1966).
Clark, L.C. Jr. et al. Perfluorocarbons having a short dwell time in the liver. Science 181, 680–682 (1973).
Moss Vision Incorporated EC Certificate 3878-2007-CE-NOR 7.0. <http://www.mossvision.co.uk/dev/certificates/CE.pdf> Originally Issued: 2007 (2012).
Castro, C.I. & Briceno, J.C. Perfluorocarbon-based oxygen carriers: review of products and trials. Artif. Organs 34, 622–634 (2010).
Kim, P., Kreder, M.J., Alvarenga, J. & Aizenberg, J. Hierarchical or not? Effect of the length scale and hierarchy of the surface roughness on omniphobicity of lubricant-infused substrates. Nano Lett. 13, 1793–1799 (2013).
Vogel, N. et al. Transparency and damage tolerance of patternable omniphobic lubricated surfaces based on inverse colloidal monolayers. Nat. Commun. 4, 2167 (2013).
Stark, A.Y. et al. Surface wettability plays a significant role in gecko adhesion underwater. Proc. Natl. Acad. Sci. USA 110, 6340–6345 (2013).
Nilsson, J. et al. A randomized study of coronary artery bypass surgery performed with the Resting Heart™ System utilizing a low vs a standard dosage of heparin. Interact. Cardiovasc. Thorac. Surg. 15, 834–839 (2012).
Gould, S.A. et al. Fluosol-DA as a red-cell substitute in acute anemia. N. Engl. J. Med. 314, 1653–1656 (1986).
Epstein, A.K. et al. Liquid-infused structured surfaces with exceptional anti-biofouling performance. Proc. Natl. Acad. Sci. USA 109, 13182–13187 (2012).
Tevaearai, H.T. et al. Trillium coating of cardiopulmonary bypass circuits improves biocompatibility. Int. J. Artif. Organs 22, 629–634 (1999).
Nilsson, L. et al. Heparin-coated equipment reduces complement activation during cardiopulmonary bypass in the pig. Artif. Organs 14, 46–48 (1990).
Hartmann, R.C. & Conley, C.L. Studies on the initiation of blood coagulation. III. The clotting properties of canine platelet-free plasma. J. Clin. Invest. 31, 685–691 (1952).
Amarnath, L.P., Srinivas, A. & Ramamurthi, A. In vitro hemocompatibility testing of UV-modified hyaluronan hydrogels. Biomaterials 27, 1416–1424 (2006).
Liu, F. & Grainger, D.W. in Comprehensive Biomaterials (ed. Ducheyne, P.) (Elsevier Science, Oxford, UK, 2011).
Toes, G.J. et al. Superhydrophobic modification fails to improve the performance of small diameter expanded polytetrafluoroethylene vascular grafts. Biomaterials 23, 255–262 (2002).
Canaud, B. et al. Pathochemical toxicity of perfluorocarbon-5070, a liquid test performance fluid previously used in dialyzer manufacturing, confirmed in animal experiment. J. Am. Soc. Nephrol. 16, 1819–1823 (2005).
Strobel, M., Lyons, C.S. & Mittal, K. Plasma Surface Modification of Polymers: Relevance to Adhesion. edn. 1. (VSP, Leiden, the Netherlands, 1994).
Schröder, K. et al. Plasma-induced surface functionalization of polymeric biomaterials in ammonia plasma. Contrib. Plasma Phys. 41, 562–572 (2001).
Ratner, B.D. Surface modification of polymers: chemical, biological and surface analytical challenges. Biosens. Bioelectron. 10, 797–804 (1995).
Zhang, Z. et al. Polybetaine modification of PDMS microfluidic devices to resist thrombus formation in whole blood. Lab Chip 13, 1963–1968 (2013).
Biological Evaluation of Medical Devices, Part 4: Selection of Tests for Interactions with Blood, 2002, Second Edition and 2006 Amendment ISO 10993-4 (Geneva, International Standards Organization, 2006).
Goodman, S.L. Sheep, pig, and human platelet–material interactions with model cardiovascular biomaterials. J. Biomed. Mater. Res. 45, 240–250 (1999).
Audran, M. et al. Determination of perfluorodecalin and perfluoro-N-methylcyclohexylpiperidine in rat blood by gas chromatography–mass spectrometry. J. Chromatogr. B Biomed. Sci. Appl. 745, 333–343 (2000).
Kang, J.H. et al. An extracorporeal blood cleansing device for sepsis therapy. Nat. Med. 10.1038/nm.3640 (14 September 2014).
Acknowledgements
This work was supported by Defense Advanced Research Projects Agency grant N66001-11-1-4180 and contract HR0011-13-C-0025, and the Wyss Institute for Biologically Inspired Engineering at Harvard University. We thank D. Super, R. Cooper, E. Murray and J. Lee for phlebotomy, T. Ferrante for assistance with fluorescence microscopy, H. Kozakewich for assistance with histology evaluation and O. Ahanotu for assistance in preparing surfaces. Scanning electron microscopy and X-ray photoelectron spectroscopy were conducted at the Center for Nanoscale Systems at Harvard University, a member of the National Nanotechnology Infrastructure Network, which is supported by the National Science Foundation (ECS-0335765).
Author information
Authors and Affiliations
Contributions
D.C.L., A.W., J.B.B., T.M.V., A.L.W., A.J., M.A., M.S., J.A. and D.E.I. designed the research. D.C.L., A.W., J.B.B., T.M.V., A.L.W., A.J., P.K., B.D.H., E.H.S., D.E.B. and S.R. performed experiments. D.C.L., A.W., J.B.B., T.M.V., A.L.W., A.J., E.H.S., M.A., M.S., J.A. and D.E.I. analyzed data. C.H., C.P.J., T.L.V., M.A. and J.A. designed, performed and analyzed the PFD leaching study. D.C.L., A.W., J.B.B., A.N., K.D., D.E.B., A.R.H., M.S. and D.E.I. designed, performed and analyzed the in vivo study. D.C.L., A.W., A.L.W., M.A., M.S., J.A. and D.E.I. wrote the paper with input from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–12, Supplementary Tables 1 and 2 and Supplementary Note 1 (PDF 1086 kb)
Supplementary Movie 1
Adhesion of human blood on untreated control acrylic. Movie of human whole blood anticoagulated with 3.2% sodium citrate)adhering to an uncoated control acrylic surface. (MOV 2345 kb)
Supplementary Movie 2
Repulsion of human blood by TLP acrylic. Movie of human whole blood (anticoagulated with 3.2% sodium citrate) repelled by a TLP acrylic surface. (MOV 1572 kb)
Supplementary Movie 3
Repulsion of human blood by TLP acrylic and polypropylene. Movie of TLP acrylic (left) and control acrylic (right) being immersed in human blood, showing no adhesion on TLP. The blood is then poured out of the TLP (left) and control (right) polypropylene (PP)tubes, again showing no adhesion of blood on the TLP surface. (MOV 62666 kb)
Supplementary Movie 4
Gecko maintains adhesion to a control acrylic cylinder as it is slowly tilted from horizontal to vertical. (MOV 10737 kb)
Supplementary Movie 5
Gecko fails to adhere to a TLP-coated acrylic cylinder as it is slowly tilted from horizontal to vertical. (MOV 6433 kb)
Rights and permissions
About this article
Cite this article
Leslie, D., Waterhouse, A., Berthet, J. et al. A bioinspired omniphobic surface coating on medical devices prevents thrombosis and biofouling. Nat Biotechnol 32, 1134–1140 (2014). https://doi.org/10.1038/nbt.3020
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt.3020
This article is cited by
-
Liquid-like polymer lubricating surfaces: Mechanism and applications
Nano Research (2024)
-
Self-anticoagulant sponge for whole blood auto-transfusion and its mechanism of coagulation factor inactivation
Nature Communications (2023)
-
An injectable and biodegradable zwitterionic gel for extending the longevity and performance of insulin infusion catheters
Nature Biomedical Engineering (2023)
-
Low friction of superslippery and superlubricity: A review
Friction (2023)
-
Oscillatory motion of viscoelastic drops on slippery lubricated surfaces
Communications Physics (2022)