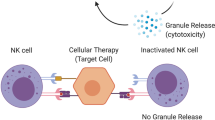
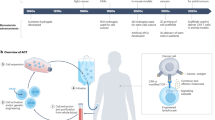
Abstract
Regenerative therapies that use allogeneic cells are likely to encounter immunological barriers similar to those that occur with transplantation of solid organs and allogeneic hematopoietic stem cells (HSCs). Decades of experience in clinical transplantation hold valuable lessons for regenerative medicine, offering approaches for developing tolerance-induction treatments relevant to cell therapies. Outside the field of solid-organ and allogeneic HSC transplantation, new strategies are emerging for controlling the immune response, such as methods based on biomaterials or mimicry of antigen-specific peripheral tolerance. Novel biomaterials can alter the behavior of cells in tissue-engineered constructs and can blunt host immune responses to cells and biomaterial scaffolds. Approaches to suppress autoreactive immune cells may also be useful in regenerative medicine. The most innovative solutions will be developed through closer collaboration among stem cell biologists, transplantation immunologists and materials scientists.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Kim Caesar/Nature Publishing Group

Kim Caesar/Nature Publishing Group
Similar content being viewed by others
References
Blazar, B.R., Murphy, W.J. & Abedi, M. Advances in graft-versus-host disease biology and therapy. Nat. Rev. Immunol. 12, 443–458 (2012).
Petersdorf, E.W. The major histocompatibility complex: a model for understanding graft-versus-host disease. Blood 122, 1863–1872 (2013).
Lakkis, F.G. & Lechler, R.I. Origin and biology of the allogeneic response. Cold Spring Harb. Perspect. Med. 3, a014993 (2013).
Bour-Jordan, H. et al. Intrinsic and extrinsic control of peripheral T-cell tolerance by costimulatory molecules of the CD28/ B7 family. Immunol. Rev. 241, 180–205 (2011).
Gill, R.G. NK cells: elusive participants in transplantation immunity and tolerance. Curr. Opin. Immunol. 22, 649–654 (2010).
Josefowicz, S.Z., Lu, L.F. & Rudensky, A.Y. Regulatory T cells: mechanisms of differentiation and function. Annu. Rev. Immunol. 30, 531–564 (2012).
Wood, K.J., Bushell, A. & Hester, J. Regulatory immune cells in transplantation. Nat. Rev. Immunol. 12, 417–430 (2012).
Calne, R.Y. et al. Cyclosporin A in patients receiving renal allografts from cadaver donors. Lancet 2, 1323–1327 (1978).
The U.S. Multicenter FK506 Liver Study Group. A comparison of tacrolimus (FK 506) and cyclosporine for immunosuppression in liver transplantation. N. Engl. J. Med. 331, 1110–1115 (1994).
Jacobson, P., Uberti, J., Davis, W. & Ratanatharathorn, V. Tacrolimus: a new agent for the prevention of graft-versus-host disease in hematopoietic stem cell transplantation. Bone Marrow Transplant. 22, 217–225 (1998).
Allison, A.C. & Eugui, E.M. Mycophenolate mofetil and its mechanisms of action. Immunopharmacology 47, 85–118 (2000).
Schlitt, H.J. et al. Replacement of calcineurin inhibitors with mycophenolate mofetil in liver-transplant patients with renal dysfunction: a randomised controlled study. Lancet 357, 587–591 (2001).
Hoda, D. et al. Sirolimus for treatment of steroid-refractory acute graft-versus-host disease. Bone Marrow Transplant. 45, 1347–1351 (2010).
Shin, H.J. et al. Rapamycin and IL-2 reduce lethal acute graft-versus-host disease associated with increased expansion of donor type CD4+CD25+Foxp3+ regulatory T cells. Blood 118, 2342–2350 (2011).
Vodanovic-Jankovic, S., Hari, P., Jacobs, P., Komorowski, R. & Drobyski, W.R. NF-kappaB as a target for the prevention of graft-versus-host disease: comparative efficacy of bortezomib and PS-1145. Blood 107, 827–834 (2006).
Koreth, J. et al. Bortezomib, tacrolimus, and methotrexate for prophylaxis of graft-versus-host disease after reduced-intensity conditioning allogeneic stem cell transplantation from HLA-mismatched unrelated donors. Blood 114, 3956–3959 (2009).
Shono, Y. et al. A small molecule c-Rel inhibitor reduces alloactivation of T-cells without compromising anti-tumor activity. Cancer Discov. 4, 578–591 (2014).
Reddy, P. et al. Histone deacetylase inhibition modulates indoleamine 2,3-dioxygenase-dependent DC functions and regulates experimental graft-versus-host disease in mice. J. Clin. Invest. 118, 2562–2573 (2008).
Strioga, M.M. et al. Therapeutic dendritic cell-based cancer vaccines: the state of the art. Crit. Rev. Immunol. 33, 489–547 (2013).
Lutz, M.B. Therapeutic potential of semi-mature dendritic cells for tolerance induction. Front. Immunol. 3, 123 (2012).
Ezzelarab, M.B. et al. Regulatory dendritic cell infusion prolongs kidney allograft survival in nonhuman primates. Am. J. Transplant. 13, 1989–2005 (2013).
Stenger, E.O., Turnquist, H.R., Mapara, M.Y. & Thomson, A.W. Dendritic cells and regulation of graft-versus-host disease and graft-versus-leukemia activity. Blood 119, 5088–5103 (2012).
Gordon, J.R., Ma, Y., Churchman, L., Gordon, S.A. & Dawicki, W. Regulatory dendritic cells for immunotherapy in immunologic diseases. Front. Immunol. 5, 7 (2014).
Rossi, R.J., Jackson, B.M., Zhang, A.H. & Scott, D.W. Tolerance induction via B-cell delivered gene therapy. Methods Mol. Biol. 900, 471–487 (2012).
Leveson-Gower, D.B. et al. Low doses of natural killer T cells provide protection from acute graft-versus-host disease via an IL-4-dependent mechanism. Blood 117, 3220–3229 (2011).
Pillai, A.B., George, T.I., Dutt, S., Teo, P. & Strober, S. Host NKT cells can prevent graft-versus-host disease and permit graft antitumor activity after bone marrow transplantation. J. Immunol. 178, 6242–6251 (2007).
Di Ianni, M. et al. Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood 117, 3921–3928 (2011).
Fan, H. et al. Regulatory T cell therapy for the induction of clinical organ transplantation tolerance. Semin. Immunol. 23, 453–461 (2011).
Joffre, O., Gorsse, N., Romagnoli, P., Hudrisier, D. & van Meerwijk, J.P. Induction of antigen-specific tolerance to bone marrow allografts with CD4+CD25+ T lymphocytes. Blood 103, 4216–4221 (2004).
Sagoo, P., Lombardi, G. & Lechler, R.I. Relevance of regulatory T cell promotion of donor-specific tolerance in solid organ transplantation. Front. Immunol. 3, 184 (2012).
Hippen, K.L. et al. Massive ex vivo expansion of human natural regulatory T cells (T(regs)) with minimal loss of in vivo functional activity. Sci. Transl. Med. 3, 83ra41 (2011).
Tang, Q. & Bluestone, J.A. Regulatory T-cell therapy in transplantation: moving to the clinic. Cold Spring Harb. Perspect. Med. 3, a015552 (2013).
Schneidawind, D., Pierini, A. & Negrin, R.S. Regulatory T cells and natural killer T cells for modulation of GVHD following allogeneic hematopoietic cell transplantation. Blood 122, 3116–3121 (2013).
Koreth, J. et al. Interleukin-2 and regulatory T cells in graft-versus-host disease. N. Engl. J. Med. 365, 2055–2066 (2011).
Choi, J. et al. In vivo administration of hypomethylating agents mitigate graft-versus-host disease without sacrificing graft-versus-leukemia. Blood 116, 129–139 (2010).
Ghobadi, A. et al. A phase I study of azacitdine after donor lymphocyte infusion for relapsed acute myeloid leukemia post allogeneic stem cell transplantation. Conference abstract of the 55th American Society of Hematology Annual Meeting (New Orleans, 2013).
Glowacki, A.J. et al. Prevention of inflammation-mediated bone loss in murine and canine periodontal disease via recruitment of regulatory lymphocytes. Proc. Natl. Acad. Sci. USA 110, 18525–18530 (2013).
Tolar, J., Le Blanc, K., Keating, A. & Blazar, B.R. Concise review: hitting the right spot with mesenchymal stromal cells. Stem Cells 28, 1446–1455 (2010).
Le Blanc, K. et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet 363, 1439–1441 (2004).
Crop, M.J. et al. Donor-derived mesenchymal stem cells suppress alloreactivity of kidney transplant patients. Transplantation 87, 896–906 (2009).
Eggenhofer, E. et al. Mesenchymal stem cells together with mycophenolate mofetil inhibit antigen presenting cell and T cell infiltration into allogeneic heart grafts. Transpl. Immunol. 24, 157–163 (2011).
Perico, N. et al. Autologous mesenchymal stromal cells and kidney transplantation: a pilot study of safety and clinical feasibility. Clin. J. Am. Soc. Nephrol. 6, 412–422 (2011).
Le Blanc, K. et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet 371, 1579–1586 (2008).
Kim, N. et al. Mesenchymal stem cells for the treatment and prevention of graft-versus-host disease: experiments and practice. Ann. Hematol. 92, 1295–1308 (2013).
Kawai, T. et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N. Engl. J. Med. 358, 353–361 (2008).
Fuchimoto, Y. et al. Mixed chimerism and tolerance without whole body irradiation in a large animal model. J. Clin. Invest. 105, 1779–1789 (2000).
Mapara, M.Y. et al. Induction of stable long-term mixed hematopoietic chimerism following nonmyeloablative conditioning with T cell-depleting antibodies, cyclophosphamide, and thymic irradiation leads to donor-specific in vitro and in vivo tolerance. Biol. Blood Marrow Transplant. 7, 646–655 (2001).
Sachs, D.H., Kawai, T. & Sykes, M. Induction of tolerance through mixed chimerism. Cold Spring Harb. Perspect. Med. 4, a015529 (2014).
Czechowicz, A., Kraft, D., Weissman, I.L. & Bhattacharya, D. Efficient transplantation via antibody-based clearance of hematopoietic stem cell niches. Science 318, 1296–1299 (2007).
Logan, A.C., Weissman, I.L. & Shizuru, J.A. The road to purified hematopoietic stem cell transplants is paved with antibodies. Curr. Opin. Immunol. 24, 640–648 (2012).
Levine, J.E. et al. Etanercept plus methylprednisolone as initial therapy for acute graft-versus-host disease. Blood 111, 2470–2475 (2008).
Wabbijn, M. et al. Ten-year follow-up of recipients of a kidney or heart transplant who received induction therapy with a monoclonal antibody against the interleukin-2 receptor. Exp. Clin. Transplant. 2, 201–207 (2004).
Xhaard, A. et al. Steroid-refractory acute GVHD: lack of long-term improved survival using new generation anticytokine treatment. Biol. Blood Marrow Transplant. 18, 406–413 (2012).
Beyersdorf, N. et al. Protection from graft-versus-host disease with a novel B7 binding site-specific mouse anti-mouse CD28 monoclonal antibody. Blood 112, 4328–4336 (2008).
Larsen, C.P. et al. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature 381, 434–438 (1996).
Li, J. et al. Roles of CD28, CTLA4, and inducible costimulator in acute graft-versus-host disease in mice. Biol. Blood Marrow Transplant. 17, 962–969 (2011).
Li, S. et al. CTLA4-Ig-based conditioning regimen to induce tolerance to cardiac allografts. J. Surg. Res. 136, 238–246 (2006).
Wallace, P.M. et al. CTLA4Ig treatment ameliorates the lethality of murine graft-versus-host disease across major histocompatibility complex barriers. Transplantation 58, 602–610 (1994).
Asberg, A. et al. Calcineurin inhibitor avoidance with daclizumab, mycophenolate mofetil, and prednisolone in DR-matched de novo kidney transplant recipients. Transplantation 82, 62–68 (2006).
Maltzman, J.S. Costimulation blockade-a double-edged sword? Am. J. Transplant. 12, 2269–2270 (2012).
Shapiro, A.M. et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N. Engl. J. Med. 343, 230–238 (2000).
Lim, F. & Sun, A.M. Microencapsulated islets as bioartificial endocrine pancreas. Science 210, 908–910 (1980).
Cui, H. et al. Long-term metabolic control of autoimmune diabetes in spontaneously diabetic nonobese diabetic mice by nonvascularized microencapsulated adult porcine islets. Transplantation 88, 160–169 (2009).
Neufeld, T. et al. The efficacy of an immunoisolating membrane system for islet xenotransplantation in minipigs. PLoS One 8, e70150 (2013).
Yap, W.T. et al. Collagen IV-modified scaffolds improve islet survival and function and reduce time to euglycemia. Tissue Eng. Part A 19, 2361–2372 (2013).
Brady, A.-C. et al. Proangiogenic hydrogels within macroporous scaffolds enhance islet engraftment in an extrahepatic site. Tissue Eng. Part A 19, 2544–2552 (2013).
Perez, V.L. et al. The anterior chamber of the eye as a clinical transplantation site for the treatment of diabetes: a study in a baboon model of diabetes. Diabetologia 54, 1121–1126 (2011).
Komori, J., Boone, L., DeWard, A., Hoppo, T. & Lagasse, E. The mouse lymph node as an ectopic transplantation site for multiple tissues. Nat. Biotechnol. 30, 976–983 (2012).
Dane, K.Y. et al. Nano-sized drug-loaded micelles deliver payload to lymph node immune cells and prolong allograft survival. J. Control. Release 156, 154–160 (2011).
Berman, D.M. et al. Mesenchymal stem cells enhance allogeneic islet engraftment in nonhuman primates. Diabetes 59, 2558–2568 (2010).
Shields, J.D., Kourtis, I.C., Tomei, A.A., Roberts, J.M. & Swartz, M.A. Induction of lymphoidlike stroma and immune escape by tumors that express the chemokine CCL21. Science 328, 749–752 (2010).
Lund, A.W. et al. VEGF-C promotes immune tolerance in B16 melanomas and cross-presentation of tumor antigen by lymph node lymphatics. Cell Reports 1, 191–199 (2012).
Hirosue, S. et al. Steady-state antigen scavenging, cross-presentation, and CD8+ T cell priming: a new role for lymphatic endothelial cells. J. Immunol. 192, 5002–5011 (2014).
Luo, X. et al. ECDI-fixed allogeneic splenocytes induce donor-specific tolerance for long-term survival of islet transplants via two distinct mechanisms. Proc. Natl. Acad. Sci. USA 105, 14527–14532 (2008).
Martin, A.J. et al. Ethylenecarbodiimide-treated splenocytes carrying male CD4 epitopes confer histocompatibility Y chromosome antigen transplant protection by inhibiting CD154 upregulation. J. Immunol. 185, 3326–3336 (2010).
Fife, B.T. et al. Insulin-induced remission in new-onset NOD mice is maintained by the PD-1-PD-L1 pathway. J. Exp. Med. 203, 2737–2747 (2006).
Liu, K. et al. Immune tolerance after delivery of dying cells to dendritic cells in situ. J. Exp. Med. 196, 1091–1097 (2002).
Kheradmand, T. et al. Permanent protection of PLG scaffold transplanted allogeneic islet grafts in diabetic mice treated with ECDI-fixed donor splenocyte infusions. Biomaterials 32, 4517–4524 (2011).
Lutterotti, A. et al. Antigen-specific tolerance by autologous myelin peptide-coupled cells: a phase 1 trial in multiple sclerosis. Sci. Translational Med. 5, 188ra175–188ra175 (2013).
Getts, D.R. et al. Microparticles bearing encephalitogenic peptides induce T-cell tolerance and ameliorate experimental autoimmune encephalomyelitis. Nat. Biotechnol. 30, 1217–1224 (2012).
Stern, J.N.H. et al. Promoting tolerance to proteolipid protein-induced experimental autoimmune encephalomyelitis through targeting dendritic cells. Proc. Natl. Acad. Sci. USA 107, 17280–17285 (2010).
Mukherjee, G. et al. DEC-205-mediated antigen targeting to steady-state dendritic cells induces deletion of diabetogenic CD8+ T cells independently of PD-1 and PD-L1. Int. Immunol. 25, 651–660 (2013).
Li, D. et al. Targeting self- and foreign antigens to dendritic cells via DC-ASGPR generates IL-10-producing suppressive CD4+ T cells. J. Exp. Med. 209, 109–121 (2012).
Tsai, S. et al. Reversal of autoimmunity by boosting memory-like autoregulatory T cells. Immunity 32, 568–580 (2010).
Kontos, S., Kourtis, I.C., Dane, K.Y. & Hubbell, J.A. Engineering antigens for in situ erythrocyte binding induces T-cell deletion. Proc. Natl. Acad. Sci. USA 110, E60–E68 (2013).
Shapiro, A.M. et al. International trial of the Edmonton protocol for islet transplantation. N. Engl. J. Med. 355, 1318–1330 (2006).
Chen, W., Sayegh, M.H. & Khoury, S.J. Mechanisms of acquired thymic tolerance in vivo: intrathymic injection of antigen induces apoptosis of thymocytes and peripheral T cell anergy. J. Immunol. 160, 1504–1508 (1998).
Cober, S.R., Randolph, M.A. & Lee, W.P. Skin allograft survival following intrathymic injection of donor bone marrow. J. Surg. Res. 85, 204–208 (1999).
Djamali, A. et al. Intrathymic injection of anti-Fas monoclonal antibody prolongs murine non-vascularized cardiac allograft survival. Transpl. Int. 17, 301–309 (2004).
Marodon, G. et al. Induction of antigen-specific tolerance by intrathymic injection of lentiviral vectors. Blood 108, 2972–2978 (2006).
Tuckett, A.Z. et al. Image-guided intrathymic injection of multipotent stem cells supports lifelong T-cell immuity and facilitates targeted immunotherapy. Blood 123, 2797–2805 (2014).
Poznansky, M.C. et al. Efficient generation of human T cells from a tissue-engineered thymic organoid. Nat. Biotechnol. 18, 729–734 (2000).
Seach, N. et al. Vascularized tissue engineering mouse chamber model supports thymopoiesis of ectopic thymus tissue grafts. Tissue Eng. Part C Methods 16, 543–551 (2010).
Clark, R.A., Yamanaka, K., Bai, M., Dowgiert, R. & Kupper, T.S. Human skin cells support thymus-independent T cell development. J. Clin. Invest. 115, 3239–3249 (2005).
Takahashi, K. & Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 (2006).
Shtrichman, R., Germanguz, I. & Itskovitz-Eldor, J. Induced pluripotent stem cells (iPSCs) derived from different cell sources and their potential for regenerative and personalized medicine. Curr. Mol. Med. 13, 792–805 (2013).
Wu, S.M. & Hochedlinger, K. Harnessing the potential of induced pluripotent stem cells for regenerative medicine. Nat. Cell Biol. 13, 497–505 (2011).
Araki, R. et al. Negligible immunogenicity of terminally differentiated cells derived from induced pluripotent or embryonic stem cells. Nature 494, 100–104 (2013).
Martin, M.J., Muotri, A., Gage, F. & Varki, A. Human embryonic stem cells express an immunogenic nonhuman sialic acid. Nat. Med. 11, 228–232 (2005).
Zhao, T., Zhang, Z.N., Rong, Z. & Xu, Y. Immunogenicity of induced pluripotent stem cells. Nature 474, 212–215 (2011).
Tang, C., Weissman, I.L. & Drukker, M. Immunogenicity of in vitro maintained and matured populations: potential barriers to engraftment of human pluripotent stem cell derivatives. Methods Mol. Biol. 1029, 17–31 (2013).
Cicciarelli, J.C., Lemp, N.A. & Kasahara, N. Prospects for designing universal stem cell lines. in The Immunological Barriers to Regenerative Medicine (ed., Fairchild, P.J.) 147–174 (Humana Press, New York, 2012).
Muraoka, N. & Ieda, M. Direct reprogramming of fibroblasts into myocytes to reverse fibrosis. Annu. Rev. Physiol. 76, 21–37 (2014).
Magro, L. et al. Imatinib mesylate as salvage therapy for refractory sclerotic chronic graft-versus-host disease. Blood 114, 719–722 (2009).
Tao, R. et al. Deacetylase inhibition promotes the generation and function of regulatory T cells. Nat. Med. 13, 1299–1307 (2007).
Vijayakrishnan, L., Venkataramanan, R. & Gulati, P. Treating inflammation with the Janus kinase inhibitor CP-690,550. Trends Pharmacol. Sci. 32, 25–34 (2011).
Evenou, J.P. et al. The potent protein kinase C-selective inhibitor AEB071 (sotrastaurin) represents a new class of immunosuppressive agents affecting early T-cell activation. J. Pharmacol. Exp. Ther. 330, 792–801 (2009).
Penack, O. et al. Inhibition of neovascularization to simultaneously ameliorate graft-vs-host disease and decrease tumor growth. J. Natl. Cancer Inst. 102, 894–908 (2010).
Shimabukuro-Vornhagen, A., Liebig, T. & von Bergwelt-Baildon, M. Statins inhibit human APC function: implications for the treatment of GVHD. Blood 112, 1544–1545 (2008).
Pruett, T.L. et al. Safety profile, pharmacokinetics, and pharmacodynamics of siplizumab, a humanized anti-CD2 monoclonal antibody, in renal allograft recipients. Transplant. Proc. 41, 3655–3661 (2009).
Adkins, D., Ratanatharathorn, V., Yang, H. & White, B. Safety profile and clinical outcomes in a phase I, placebo-controlled study of siplizumab in acute graft-versus-host disease. Transplantation 88, 198–202 (2009).
Christopeit, M. et al. Rituximab reduces the incidence of acute graft-versus-host disease. Blood 113, 3130–3131 (2009).
Schub, N. et al. Therapy of steroid-refractory acute GVHD with CD52 antibody alemtuzumab is effective. Bone Marrow Transplant. 46, 143–147 (2011).
Garcia-Cadenas, I. et al. Updated experience with inolimomab as treatment for corticosteroid-refractory acute graft-versus-host disease. Biol. Blood Marrow Transplant. 19, 435–439 (2013).
Drobyski, W.R. et al. Tocilizumab for the treatment of steroid refractory graft-versus-host disease. Biol. Blood Marrow Transplant. 17, 1862–1868 (2011).
Chung, B. et al. Prevention of graft-versus-host disease by anti IL-7Ralpha antibody. Blood 110, 2803–2810 (2007).
Mai, H.L. et al. IL-7 receptor blockade following T cell depletion promotes long-term allograft survival. J. Clin. Invest. 124, 1723–1733 (2014).
Jang, M.S. et al. A blocking anti-CD28-specific antibody induces long-term heart allograft survival by suppression of the PKC theta-JNK signal pathway. Transplantation 85, 1051–1055 (2008).
Li, N. et al. Blockade of CD28 by a synthetical peptoid inhibits T-cell proliferation and attenuates graft-versus-host disease. Cell. Mol. Immunol. 7, 133–142 (2010).
Page, A. et al. CD40 blockade combines with CTLA4Ig and sirolimus to produce mixed chimerism in an MHC-defined rhesus macaque transplant model. Am. J. Transplant. 12, 115–125 (2012).
Taylor, P.A. et al. Targeting of inducible costimulator (ICOS) expressed on alloreactive T cells down-regulates graft-versus-host disease (GVHD) and facilitates engraftment of allogeneic bone marrow (BM). Blood 105, 3372–3380 (2005).
Trzonkowski, P. et al. First-in-man clinical results of the treatment of patients with graft versus host disease with human ex vivo expanded CD4+CD25+CD127- T regulatory cells. Clin. Immunol. 133, 22–26 (2009).
Highfill, S.L. et al. Bone marrow myeloid-derived suppressor cells (MDSCs) inhibit graft-versus-host disease (GVHD) via an arginase-1-dependent mechanism that is up-regulated by interleukin-13. Blood 116, 5738–5747 (2010).
Acknowledgements
We thank A.Z. Tuckett for valuable feedback and her input in the design of Figure 3.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Jeffrey Hubbell wishes to declare that he is founder and shareholder of Anokion SA, which is commercializing the technology described in the manuscript regarding binding antigens to erythrocytes for tolerization.
Rights and permissions
About this article
Cite this article
Zakrzewski, J., van den Brink, M. & Hubbell, J. Overcoming immunological barriers in regenerative medicine. Nat Biotechnol 32, 786–794 (2014). https://doi.org/10.1038/nbt.2960
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt.2960
This article is cited by
-
Decellularized Tissue-Induced Cellular Recruitment for Tissue Engineering and Regenerative Medicine
Annals of Biomedical Engineering (2023)
-
The Progress of Stem Cell Therapy in Myocardial-Infarcted Heart Regeneration: Cell Sheet Technology
Tissue Engineering and Regenerative Medicine (2022)
-
Efficient hepatic differentiation and regeneration potential under xeno-free conditions using mass-producible amnion-derived mesenchymal stem cells
Stem Cell Research & Therapy (2021)
-
A biomaterial-based vaccine eliciting durable tumour-specific responses against acute myeloid leukaemia
Nature Biomedical Engineering (2020)
-
A strategy to protect off-the-shelf cell therapy products using virus-specific T-cells engineered to eliminate alloreactive T-cells
Journal of Translational Medicine (2019)