Abstract
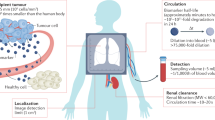
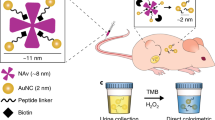
Biomarkers are becoming increasingly important in the clinical management of complex diseases, yet our ability to discover new biomarkers remains limited by our dependence on endogenous molecules. Here we describe the development of exogenously administered 'synthetic biomarkers' composed of mass-encoded peptides conjugated to nanoparticles that leverage intrinsic features of human disease and physiology for noninvasive urinary monitoring. These protease-sensitive agents perform three functions in vivo: they target sites of disease, sample dysregulated protease activities and emit mass-encoded reporters into host urine for multiplexed detection by mass spectrometry. Using mouse models of liver fibrosis and cancer, we show that these agents can noninvasively monitor liver fibrosis and resolution without the need for invasive core biopsies and substantially improve early detection of cancer compared with current clinically used blood biomarkers. This approach of engineering synthetic biomarkers for multiplexed urinary monitoring should be broadly amenable to additional pathophysiological processes and point-of-care diagnostics.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Sawyers, C.L. The cancer biomarker problem. Nature 452, 548–552 (2008).
Hanash, S.M., Pitteri, S.J. & Faca, V.M. Mining the plasma proteome for cancer biomarkers. Nature 452, 571–579 (2008).
Sreekumar, A. et al. Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature 457, 910–914 (2009).
Findeisen, P. & Neumaier, M. Functional protease profiling for diagnosis of malignant disease. Proteomics Clin. Appl. 6, 60–78 (2012).
Surinova, S. et al. On the development of plasma protein biomarkers. J. Proteome Res. 10, 5–16 (2011).
Schwarzenbach, H., Hoon, D.S.B. & Pantel, K. Cell-free nucleic acids as biomarkers in cancer patients. Nat. Rev. Cancer 11, 426–437 (2011).
Moon, P.-G., You, S., Lee, J.-E., Hwang, D. & Baek, M.-C. Urinary exosomes and proteomics. Mass Spectrom. Rev. 30, 1185–1202 (2011).
Nagrath, S. et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature 450, 1235–1239 (2007).
Lutz, A.M., Willmann, J.K., Cochran, F.V., Ray, P. & Gambhir, S.S. Cancer screening: a mathematical model relating secreted blood biomarker levels to tumor sizes. PLoS Med. 5, e170 (2008).
Haun, J.B. et al. Micro-NMR for rapid molecular analysis of human tumor samples. Sci. Transl. Med. 3, 71ra16 (2011).
Edgington, L.E., Verdoes, M. & Bogyo, M. Functional imaging of proteases: recent advances in the design and application of substrate-based and activity-based probes. Curr. Opin. Chem. Biol. 15, 798–805 (2011).
Nomura, D.K., Dix, M.M. & Cravatt, B.F. Activity-based protein profiling for biochemical pathway discovery in cancer. Nat. Rev. Cancer 10, 630–638 (2010).
Hilderbrand, S.A. & Weissleder, R. Near-infrared fluorescence: application to in vivo molecular imaging. Curr. Opin. Chem. Biol. 14, 71–79 (2010).
Braet, F. & Wisse, E. Structural and functional aspects of liver sinusoidal endothelial cell fenestrae: a review. Comp. Hepatol. 1, 1 (2002).
Jain, R.K. & Stylianopoulos, T. Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol. 7, 653–664 (2010).
López-Otín, C. & Bond, J.S. Proteases: multifunctional enzymes in life and disease. J. Biol. Chem. 283, 30433–30437 (2008).
Schuppan, D. & Afdhal, N.H. Liver cirrhosis. Lancet 371, 838–851 (2008).
Hori, S.S. & Gambhir, S.S. Mathematical model identifies blood biomarker-based early cancer detection strategies and limitations. Sci. Transl. Med. 3, 109ra116 (2011).
Bremer, C., Tung, C.H. & Weissleder, R. In vivo molecular target assessment of matrix metalloproteinase inhibition. Nat. Med. 7, 743–748 (2001).
Kridel, S.J. et al. A unique substrate binding mode discriminates membrane type-1 matrix metalloproteinase from other matrix metalloproteinases. J. Biol. Chem. 277, 23788–23793 (2002).
Lutolf, M.P. et al. Repair of bone defects using synthetic mimetics of collagenous extracellular matrices. Nat. Biotechnol. 21, 513–518 (2003).
Mahmood, U. & Weissleder, R. Near-infrared optical imaging of proteases in cancer. Mol. Cancer Ther. 2, 489–496 (2003).
Turk, B.E., Huang, L.L., Piro, E.T. & Cantley, L.C. Determination of protease cleavage site motifs using mixture-based oriented peptide libraries. Nat. Biotechnol. 19, 661–667 (2001).
Park, J.-H. et al. Systematic surface engineering of magnetic nanoworms for in vivo tumor targeting. Small 5, 694–700 (2009).
Fickert, P. et al. A new xenobiotic-induced mouse model of sclerosing cholangitis and biliary fibrosis. Am. J. Pathol. 171, 525–536 (2007).
Morris, T.A. et al. Urine and plasma levels of fibrinopeptide b in patients with deep vein thrombosis and pulmonary embolism. Thromb. Res. 110, 159–165 (2003).
Choi, H.S. et al. Renal clearance of quantum dots. Nat. Biotechnol. 25, 1165–1170 (2007).
Park, J.-H. et al. Magnetic iron oxide nanoworms for tumor targeting and imaging. Adv. Mater. 20, 1630–1635 (2008).
Villanueva, J. et al. Differential exoprotease activities confer tumor-specific serum peptidome patterns. J. Clin. Invest. 116, 271–284 (2006).
Villanueva, J. et al. A sequence-specific exopeptidase activity test (sseat) for “functional” biomarker discovery. Mol. Cell. Proteomics 7, 509–518 (2008).
Brown, B.B., Wagner, D.S. & Geysen, H.M. A single-bead decode strategy using electrospray ionization mass spectrometry and a new photolabile linker: 3-amino-3-(2-nitrophenyl)propionic acid. Mol. Divers. 1, 4–12 (1995).
Ross, P.L. et al. Multiplexed protein quantitation in Saccharomyces cerevisiae using amine-reactive isobaric tagging reagents.ralph. Mol. Cell. Proteomics 3, 1154–1169 (2004).
Thompson, A. et al. Tandem mass tags: a novel quantification strategy for comparative analysis of complex protein mixtures by MS/MS. Anal. Chem. 75, 1895–1904 (2003); erratum 75, 4942 (2003); erratum 78, 4235 (2006).
Rockey, D.C. et al. Liver biopsy. Hepatology 49, 1017–1044 (2009).
Popov, Y. & Schuppan, D. Targeting liver fibrosis: strategies for development and validation of antifibrotic therapies. Hepatology 50, 1294–1306 (2009).
Bedossa, P., Dargère, D. & Paradis, V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology 38, 1449–1457 (2003).
Mischak, H. et al. Recommendations for biomarker identification and qualification in clinical proteomics. Sci. Transl. Med. 2, 46ps42 (2010).
Popov, Y., Patsenker, E., Fickert, P., Trauner, M. & Schuppan, D. Mdr2 abcb4−/− mice spontaneously develop severe biliary fibrosis via massive dysregulation of pro- and antifibrogenic genes. J. Hepatol. 43, 1045–1054 (2005).
Etzioni, R. et al. The case for early detection. Nat. Rev. Cancer 3, 243–252 (2003).
D'Souza, A.L. et al. A strategy for blood biomarker amplification and localization using ultrasound. Proc. Natl. Acad. Sci. USA 106, 17152–17157 (2009).
Dekker, L.J.M. et al. Differential expression of protease activity in serum samples of prostate carcinoma patients with metastases. Proteomics 10, 2348–2358 (2010).
Ruoslahti, E., Bhatia, S.N. & Sailor, M.J. Targeting of drugs and nanoparticles to tumors. J. Cell Biol. 188, 759–768 (2010).
Sugahara, K.N. et al. Coadministration of a tumor-penetrating peptide enhances the efficacy of cancer drugs. Science 328, 1031–1035 (2010).
Kulasingam, V., Pavlou, M.P. & Diamandis, E.P. Integrating high-throughput technologies in the quest for effective biomarkers for ovarian cancer. Nat. Rev. Cancer 10, 371–378 (2010).
Acknowledgements
We thank R. Cook, N. Schiller and M. Brown (Swanson Biotechnology Center (SBC), Massachusetts Institute of Technology (MIT)) for peptide synthesis and tissue sectioning, C. Whittaker (SBC, MIT) for bioinformatic insight, R. Tomaino (Harvard Taplin mass spectrometry facility) for mass spectrometry analysis, F. Giammo and D. Kim (MIT) for initial probe work and urine purification, S. Carr (Broad Institute of MIT/Harvard) for mass spectrometry expertise and M. Sailor (University of California San Diego (UCSD)), J. Park (UCSD) and S. Friedman (Mount Sinai School of Medicine) for insightful discussions. This work was funded by the US National Institutes of Health (Bioengineering Research Partnership: R01 CA124427 to S.N.B.), the Kathy and Curt Marble Cancer Research Fund to S.N.B. and US National Institutes of Health grants U19 AI066313 and 1R21 DK075857 to D.S. G.A.K. is supported by the Ruth L. Kirschstein National Research Service Award (F32CA159496-01). S.N.B. is a Howard Hughes Institute Investigator.
Author information
Authors and Affiliations
Contributions
G.A.K., G.v.M. and S.N.B. conceived the study and designed the experiments. G.v.M. and S.M. performed the in vitro substrate screen and initial pharmacokinetic studies. G.A.K. developed the mass-encoding scheme. G.A.K., G.M. and O.A. performed the fibrosis experiments. Y.P., D.Y.S., S.B.L. and D.S. developed and provided expertise for the fibrosis progression and reversal protocols, and performed fibrosis quantification assays. G.A.K. and A.D.W. performed cancer experiments. G.A.K. and I.A.P. collected in vivo mass spectrometry data. G.A.K., G.v.M. and S.N.B. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–19 (PDF 1334 kb)
Rights and permissions
About this article
Cite this article
Kwong, G., von Maltzahn, G., Murugappan, G. et al. Mass-encoded synthetic biomarkers for multiplexed urinary monitoring of disease. Nat Biotechnol 31, 63–70 (2013). https://doi.org/10.1038/nbt.2464
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt.2464
This article is cited by
-
A renal clearable fluorogenic probe for in vivo β-galactosidase activity detection during aging and senolysis
Nature Communications (2024)
-
CRISPR-Cas-amplified urinary biomarkers for multiplexed and portable cancer diagnostics
Nature Nanotechnology (2023)
-
Renal clearable polyfluorophore nanosensors for early diagnosis of cancer and allograft rejection
Nature Materials (2022)
-
Urinary detection of early responses to checkpoint blockade and of resistance to it via protease-cleaved antibody-conjugated sensors
Nature Biomedical Engineering (2022)
-
The Need to Pair Molecular Monitoring Devices with Molecular Imaging to Personalize Health
Molecular Imaging and Biology (2022)