Abstract
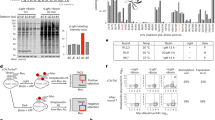
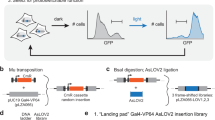
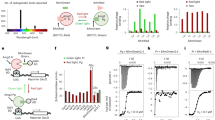
Protein-protein interactions are essential for many cellular processes. We have developed a technology called light-activated dimerization (LAD) to artificially induce protein hetero- and homodimerization in live cells using light. Using the FKF1 and GIGANTEA (GI) proteins of Arabidopsis thaliana, we have generated protein tags whose interaction is controlled by blue light. We demonstrated the utility of this system with LAD constructs that can recruit the small G-protein Rac1 to the plasma membrane and induce the local formation of lamellipodia in response to focal illumination. We also generated a light-activated transcription factor by fusing domains of GI and FKF1 to the DNA binding domain of Gal4 and the transactivation domain of VP16, respectively, showing that this technology is easily adapted to other systems. These studies set the stage for the development of light-regulated signaling molecules for controlling receptor activation, synapse formation and other signaling events in organisms.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Boyden, E.S., Zhang, F., Bamberg, E., Nagel, G. & Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 8, 1263–1268 (2005).
Schroder-Lang, S. et al. Fast manipulation of cellular cAMP level by light in vivo. Nat. Methods 4, 39–42 (2007).
Melyan, Z., Tarttelin, E.E., Bellingham, J., Lucas, R.J. & Hankins, M.W. Addition of human melanopsin renders mammalian cells photoresponsive. Nature 433, 741–745 (2005).
Airan, R.D., Thompson, K.R., Fenno, L.E., Bernstein, H. & Deisseroth, K. Temporally precise in vivo control of intracellular signalling. Nature 458, 1025–1029 (2009).
Sawa, M., Nusinow, D.A., Kay, S.A. & Imaizumi, T. FKF1 and GIGANTEA complex formation is required for day-length measurement in Arabidopsis. Science 318, 261–265 (2007).
Nelson, D.C., Lasswell, J., Rogg, L.E., Cohen, M.A. & Bartel, B. FKF1, a clock-controlled gene that regulates the transition to flowering in Arabidopsis. Cell 101, 331–340 (2000).
Imaizumi, T., Tran, H.G., Swartz, T.E., Briggs, W.R. & Kay, S.A. FKF1 is essential for photoperiodic-specific light signalling in Arabidopsis. Nature 426, 302–306 (2003).
Zikihara, K. et al. Photoreaction cycle of the light, oxygen, and voltage domain in FKF1 determined by low-temperature absorption spectroscopy. Biochemistry 45, 10828–10837 (2006).
Huq, E., Tepperman, J.M. & Quail, P.H. GIGANTEA is a nuclear protein involved in phytochrome signaling in Arabidopsis. Proc. Natl. Acad. Sci. USA 97, 9789–9794 (2000).
Nakai, K. & Horton, P. PSORT: a program for detecting sorting signals in proteins and predicting their subcellular localization. Trends Biochem. Sci. 24, 34–36 (1999).
Kalderon, D., Roberts, B.L., Richardson, W.D. & Smith, A.E. A short amino acid sequence able to specify nuclear location. Cell 39, 499–509 (1984).
Magee, T. & Marshall, C. New insights into the interaction of Ras with the plasma membrane. Cell 98, 9–12 (1999).
Kim, W.Y. et al. ZEITLUPE is a circadian photoreceptor stabilized by GIGANTEA in blue light. Nature 449, 356–360 (2007).
Kasahara, M. et al. Photochemical properties of the flavin mononucleotide-binding domains of the phototropins from Arabidopsis, rice, and Chlamydomonas reinhardtii. Plant Physiol. 129, 762–773 (2002).
Ridley, A.J. & Hall, A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell 70, 389–399 (1992).
Xu, X., Barry, D.C., Settleman, J., Schwartz, M.A. & Bokoch, G.M. Differing structural requirements for GTPase-activating protein responsiveness and NADPH oxidase activation by Rac. J. Biol. Chem. 269, 23569–23574 (1994).
Inoue, T., Heo, W.D., Grimley, J.S., Wandless, T.J. & Meyer, T. An inducible translocation strategy to rapidly activate and inhibit small GTPase signaling pathways. Nat. Methods 2, 415–418 (2005).
Wen, W., Meinkoth, J.L., Tsien, R.Y. & Taylor, S.S. Identification of a signal for rapid export of proteins from the nucleus. Cell 82, 463–473 (1995).
Giniger, E., Varnum, S.M. & Ptashne, M. Specific DNA binding of GAL4, a positive regulatory protein of yeast. Cell 40, 767–774 (1985).
Sadowski, I., Ma, J., Triezenberg, S. & Ptashne, M. GAL4–VP16 is an unusually potent transcriptional activator. Nature 335, 563–564 (1988).
Kaang, B.K., Kandel, E.R. & Grant, S.G. Activation of cAMP-responsive genes by stimuli that produce long-term facilitation in Aplysia sensory neurons. Neuron 10, 427–435 (1993).
Spencer, D.M., Wandless, T.J., Schreiber, S.L. & Crabtree, G.R. Controlling signal transduction with synthetic ligands. Science 262, 1019–1024 (1993).
Farrar, M.A., Alberol, I. & Perlmutter, R.M. Activation of the Raf-1 kinase cascade by coumermycin-induced dimerization. Nature 383, 178–181 (1996).
Wiens, K.M., Lin, H. & Liao, D. Rac1 induces the clustering of AMPA receptors during spinogenesis. J. Neurosci. 25, 10627–10636 (2005).
Walmsley, M.J. et al. Critical roles for Rac1 and Rac2 GTPases in B cell development and signaling. Science 302, 459–462 (2003).
Satoh, M. et al. Requirement of Rac1 in the development of cardiac hypertrophy. Proc. Natl. Acad. Sci. USA 103, 7432–7437 (2006).
Liu, M., Komiyama, M. & Asanuma, H. Design of light-switchable phage promoter for efficient photo-regulation of gene-expression. Nucleic Acids Symp. Ser. (Oxf) 49, 283–284 (2005).
Shimizu-Sato, S., Huq, E., Tepperman, J.M. & Quail, P.H. A light-switchable gene promoter system. Nat. Biotechnol. 20, 1041–1044 (2002).
Dyer, B.W., Ferrer, F.A., Klinedinst, D.K. & Rodriguez, R. A noncommercial dual luciferase enzyme assay system for reporter gene analysis. Anal. Biochem. 282, 158–161 (2000).
Acknowledgements
We would like to thank T. Imaizumi (Scripps Research Institute) for providing FKF1 and GI plasmids; T.-S. Tseng and W.R. Briggs (Carnegie Institution, Stanford University) for providing phototropin plasmids; Y. Gotoh (University of Tokyo) for providing plasmids encoding human Rac1 mutants; and M. Endo and S. Sugano for helpful advice. This study is supported by an National Institutes of Health Pioneer Award, the Simons Foundation and by gifts from L. Miller, B. and F. Horowitz and M. MCafferey to R.E.D., the Japan Society for the Promotion of Science Postdoctoral Fellowships for Research Abroad to M.Y. and Stanford Institutes of Medicine Summer Research Program to B.H.
Author information
Authors and Affiliations
Contributions
M.Y. and R.E.D. designed research and wrote manuscript; M.Y. performed plasmid construction, live-cell imaging and data analysis; M.Y. and A.M.S. conducted mutagenesis of FKF1; M.Y. and B.H. performed immunocytochemistry and luciferase assays.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figs. 1–8 and Supplementary Tables 1, 2 (PDF 963 kb)
Rights and permissions
About this article
Cite this article
Yazawa, M., Sadaghiani, A., Hsueh, B. et al. Induction of protein-protein interactions in live cells using light. Nat Biotechnol 27, 941–945 (2009). https://doi.org/10.1038/nbt.1569
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt.1569
This article is cited by
-
Designer installation of a substrate recruitment domain to tailor enzyme specificity
Nature Chemical Biology (2023)
-
A small and highly sensitive red/far-red optogenetic switch for applications in mammals
Nature Biotechnology (2022)
-
Temporal induction of Lhx8 by optogenetic control system for efficient bone regeneration
Stem Cell Research & Therapy (2021)
-
A guide to the optogenetic regulation of endogenous molecules
Nature Methods (2021)
-
A LexA-based yeast two-hybrid system for studying light-switchable interactions of phytochromes with their interacting partners
aBIOTECH (2021)