Abstract
Vesicular carriers transport proteins and lipids from one organelle to another, recognizing specific identifiers for the donor and acceptor membranes. Two important identifiers are phosphoinositides and GTP-bound GTPases, which provide well-defined but mutable labels. Phosphatidylinositol and its phosphorylated derivatives are present on the cytosolic faces of most cellular membranes1,2. Reversible phosphorylation of its headgroup produces seven distinct phosphoinositides. In endocytic traffic, phosphatidylinositol-4,5-biphosphate marks the plasma membrane, and phosphatidylinositol-3-phosphate and phosphatidylinositol-4-phosphate mark distinct endosomal compartments2,3. It is unknown what sequence of changes in lipid content confers on the vesicles their distinct identity at each intermediate step. Here we describe ‘coincidence-detecting’ sensors that selectively report the phosphoinositide composition of clathrin-associated structures, and the use of these sensors to follow the dynamics of phosphoinositide conversion during endocytosis. The membrane of an assembling coated pit, in equilibrium with the surrounding plasma membrane, contains phosphatidylinositol-4,5-biphosphate and a smaller amount of phosphatidylinositol-4-phosphate. Closure of the vesicle interrupts free exchange with the plasma membrane. A substantial burst of phosphatidylinositol-4-phosphate immediately after budding coincides with a burst of phosphatidylinositol-3-phosphate, distinct from any later encounter with the phosphatidylinositol-3-phosphate pool in early endosomes; phosphatidylinositol-3,4-biphosphate and the GTPase Rab5 then appear and remain as the uncoating vesicles mature into Rab5-positive endocytic intermediates. Our observations show that a cascade of molecular conversions, made possible by the separation of a vesicle from its parent membrane, can label membrane-traffic intermediates and determine their destinations.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Vance, J. E. & Steenbergen, R. Metabolism and functions of phosphatidylserine. Prog. Lipid Res. 44, 207–234 (2005)
Balla, T. Phosphoinositides: tiny lipids with giant impact on cell regulation. Physiol. Rev. 93, 1019–1137 (2013)
Di Paolo, G. & De Camilli, P. Phosphoinositides in cell regulation and membrane dynamics. Nature 443, 651–657 (2006)
Massol, R. H., Boll, W., Griffin, A. M. & Kirchhausen, T. A burst of auxilin recruitment determines the onset of clathrin-coated vesicle uncoating. Proc. Natl Acad. Sci. USA 103, 10265–10270 (2006)
Mills, I. G. et al. EpsinR: an AP1/clathrin interacting protein involved in vesicle trafficking. J. Cell Biol. 160, 213–222 (2003)
Messa, M. et al. Epsin deficiency impairs endocytosis by stalling the actin-dependent invagination of endocytic clathrin-coated pits. eLife 3, e03311 (2014)
Lee, D. W., Wu, X., Eisenberg, E. & Greene, L. E. Recruitment dynamics of GAK and auxilin to clathrin-coated pits during endocytosis. J. Cell Sci. 119, 3502–3512 (2006)
Guan, R., Dai, H., Harrison, S. C. & Kirchhausen, T. Structure of the PTEN-like region of auxilin, a detector of clathrin-coated vesicle budding. Structure 18, 1191–1198 (2010)
Fotin, A. et al. Structure of an auxilin-bound clathrin coat and its implications for the mechanism of uncoating. Nature 432, 649–653 (2004)
Aguet, F., Antonescu, C. N., Mettlen, M., Schmid, S. L. & Danuser, G. Advances in analysis of low signal-to-noise images link dynamin and AP2 to the functions of an endocytic checkpoint. Dev. Cell 26, 279–291 (2013)
Chen, B. C. et al. Lattice light-sheet microscopy: imaging molecules to embryos at high spatiotemporal resolution. Science 346, 1257998 (2014)
Várnai, P. & Balla, T. Visualization of phosphoinositides that bind pleckstrin homology domains: calcium- and agonist-induced dynamic changes and relationship to myo-[3H]inositol-labeled phosphoinositide pools. J. Cell Biol. 143, 501–510 (1998)
Temmerman, K. et al. A direct role for phosphatidylinositol-4,5-bisphosphate in unconventional secretion of fibroblast growth factor 2. Traffic 9, 1204–1217 (2008)
Idevall-Hagren, O., Dickson, E. J., Hille, B., Toomre, D. K. & De Camilli, P. Optogenetic control of phosphoinositide metabolism. Proc. Natl Acad. Sci. USA 109, E2316–E2323 (2012)
Boucrot, E., Saffarian, S., Massol, R., Kirchhausen, T. & Ehrlich, M. Role of lipids and actin in the formation of clathrin-coated pits. Exp. Cell Res. 312, 4036–4048 (2006)
Zoncu, R. et al. Loss of endocytic clathrin-coated pits upon acute depletion of phosphatidylinositol 4,5-bisphosphate. Proc. Natl Acad. Sci. USA 104, 3793–3798 (2007)
Gillooly, D. J. et al. Localization of phosphatidylinositol 3-phosphate in yeast and mammalian cells. EMBO J. 19, 4577–4588 (2000)
Hammond, G. R., Machner, M. P. & Balla, T. A novel probe for phosphatidylinositol 4-phosphate reveals multiple pools beyond the Golgi. J. Cell Biol. 205, 113–126 (2014)
Zhu, Y. et al. Structural mechanism of host Rab1 activation by the bifunctional Legionella type IV effector SidM/DrrA. Proc. Natl Acad. Sci. USA 107, 4699–4704 (2010)
Posor, Y. et al. Spatiotemporal control of endocytosis by phosphatidylinositol-3,4-bisphosphate. Nature 499, 233–237 (2013)
Cocucci, E., Gaudin, R. & Kirchhausen, T. Dynamin recruitment and membrane scission at the neck of a clathrin-coated pit. Mol. Biol. Cell 25, 3595–3609 (2014)
Schmid, S. L. & Frolov, V. A. Dynamin: functional design of a membrane fission catalyst. Annu. Rev. Cell Dev. Biol. 27, 79–105 (2011)
Oikawa, T., Itoh, T. & Takenawa, T. Sequential signals toward podosome formation in NIH-src cells. J. Cell Biol. 182, 157–169 (2008)
De Matteis, M. A., Staiano, L., Emma, F. & Devuyst, O. The 5-phosphatase OCRL in Lowe syndrome and Dent disease 2. Nat. Rev. Nephrol. 13, 455–470 (2017)
Cremona, O. et al. Essential role of phosphoinositide metabolism in synaptic vesicle recycling. Cell 99, 179–188 (1999)
Villaseñor, R., Kalaidzidis, Y. & Zerial, M. Signal processing by the endosomal system. Curr. Opin. Cell Biol. 39, 53–60 (2016)
Chou, Y. Y. et al. Identification and characterization of a novel broad-spectrum virus entry inhibitor. J. Virol. 90, 4494–4510 (2016)
Bojjireddy, N. et al. Pharmacological and genetic targeting of the PI4KA enzyme reveals its important role in maintaining plasma membrane phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate levels. J. Biol. Chem. 289, 6120–6132 (2014)
Dowdle, W. E. et al. Selective VPS34 inhibitor blocks autophagy and uncovers a role for NCOA4 in ferritin degradation and iron homeostasis in vivo. Nat. Cell Biol. 16, 1069–1079 (2014)
Bago, R. et al. Characterization of VPS34-IN1, a selective inhibitor of Vps34, reveals that the phosphatidylinositol 3-phosphate-binding SGK3 protein kinase is a downstream target of class III phosphoinositide 3-kinase. Biochem. J. 463, 413–427 (2014)
Grimm, J. B. et al. A general method to improve fluorophores for live-cell and single-molecule microscopy. Nat. Methods 12, 244–250 (2015)
Gozani, O. et al. The PHD finger of the chromatin-associated protein ING2 functions as a nuclear phosphoinositide receptor. Cell 114, 99–111 (2003)
Várnai, P., Rother, K. I. & Balla, T. Phosphatidylinositol 3-kinase-dependent membrane association of the Bruton’s tyrosine kinase pleckstrin homology domain visualized in single living cells. J. Biol. Chem. 274, 10983–10989 (1999)
Yoshioka, K. et al. Endothelial PI3K-C2α, a class II PI3K, has an essential role in angiogenesis and vascular barrier function. Nat. Med. 18, 1560–1569 (2012)
Cocucci, E., Aguet, F., Boulant, S. & Kirchhausen, T. The first five seconds in the life of a clathrin-coated pit. Cell 150, 495–507 (2012)
Lawe, D. C., Patki, V., Heller-Harrison, R., Lambright, D. & Corvera, S. The FYVE domain of early endosome antigen 1 is required for both phosphatidylinositol 3-phosphate and Rab5 binding. Critical role of this dual interaction for endosomal localization. J. Biol. Chem. 275, 3699–3705 (2000)
Forozan, F. et al. Molecular cytogenetic analysis of 11 new breast cancer cell lines. Br. J. Cancer 81, 1328–1334 (1999)
Nández, R. et al. A role of OCRL in clathrin-coated pit dynamics and uncoating revealed by studies of Lowe syndrome cells. eLife 3, e02975 (2014)
Suchy, S. F. & Nussbaum, R. L. The deficiency of PIP2 5-phosphatase in Lowe syndrome affects actin polymerization. Am. J. Hum. Genet. 71, 1420–1427 (2002)
Ran, F. A. et al. Genome engineering using the CRISPR–Cas9 system. Nat. Protocols 8, 2281–2308 (2013)
Sanjana, N. E. et al. A transcription activator-like effector toolbox for genome engineering. Nat. Protocols 7, 171–192 (2012)
Shalem, O. et al. Genome-scale CRISPR–Cas9 knockout screening in human cells. Science 343, 84–87 (2014)
Kasai, R. S. et al. Full characterization of GPCR monomer–dimer dynamic equilibrium by single molecule imaging. J. Cell Biol. 192, 463–480 (2011)
Zhang, K., Chowdary, P. D. & Cui, B. Visualizing directional Rab7 and TrkA cotrafficking in axons by pTIRF microscopy. Methods Mol. Biol. 1298, 319–329 (2015)
Kadlecova, Z. et al. Regulation of clathrin-mediated endocytosis by hierarchical allosteric activation of AP2. J. Cell Biol. 216, 167–179 (2017)
Aguet, F. et al. Membrane dynamics of dividing cells imaged by lattice light-sheet microscopy. Mol. Biol. Cell 27, 3418–3435 (2016)
Cohen, J. Statistical Power Analysis for the Behavioral Sciences 2nd edn (L. Erlbaum Associates, 1988)
Acknowledgements
We thank D. Alessi, T. Balla, P. De Camilli, O. Gozani, L. Lavis, H. Stenmark, T. Takenawa and Y. Takuwa for reagents; J. R. Houser for maintaining the TIRF and spinning-disk microscopes; J. England for advice and support; members of our laboratory for help and encouragement; and in particular S. C. Harrison for discussions and editorial help. R.M. was supported by a National Defense Science and Engineering Graduate (NDSEG) Fellowship from the DoD Air Force Office of Scientific Research and E.S. by the National Natural Science Foundation of China (31770900, 31270884, 30900268), the Beijing Natural Science Foundation (5122026, 5092017) and the Youth Innovation Promotion Association of the Chinese Academy of Sciences (2011087). S.U. is a Fellow at the Image and Data Analysis core at Harvard Medical School and thanks H. Elliott and D. Richmond for discussions, and acknowledges the MATLAB code repository received from the Computational Image Analysis Workshop supported by NIH grant GM103792. T.K. acknowledges support from the Janelia Visitor Program and thanks E. Betzig, E. Marino, T. Liu and W. Legant for help and advice in constructing and installing the lattice light-sheet microscope. Construction of the lattice light-sheet microscope was supported by grants from Biogen and Ionis Pharmaceuticals to T.K. The research was supported by NIH grant NIH R01 GM075252 to T.K.
Author information
Authors and Affiliations
Contributions
K.H. and T.K. designed experiments; K.H. generated the probe constructs, and performed and analysed the experiments using TIRF and spinning-disk confocal microscopy; S.U., K.H. and W.S. performed and analysed the experiments using the lattice light-sheet microscope; R.G. established gene-editing protocols; R.G., K.H., and W.W. designed constructs for gene-editing; K.H., E.S., S.D., R.G., W.W. and M.M. generated the gene-edited cell lines; B.R.C. purified the recombinant proteins and carried out the in vitro lipid-binding assay; K.H., R.M. and T.K. discussed the results and contributed to the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
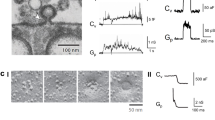
Extended Data Figure 1 Characterization of gene-edited SUM159 cells expressing clathrin light chain A and minimal requirements for the targeting specificity of the Aux1-based PtdIns(4,5)P2 sensor to endocytic coated pits and vesicles.
a, Schematic representation of the constructs EGFP–Aux1, EGFP–Aux1(1–814), and EGFP–Aux1(420–814) used for transient expression in CLTA–TagRFP+/+ cells. The representative kymographs are from 300-s time series imaged every 1 s by TIRF microscopy at the bottom surface of the cells. The kymographs were shifted laterally by six pixels. Scale bars, 5 μm. The plots show averaged fluorescence intensity traces (mean ± s.e.m.) of CLTA–TagRFP (red) and the Aux1 constructs (green) from endocytic clathrin-coated pits and vesicles automatically identified using the 2D tracking software in 11, 6 and 12 cells, respectively, and then grouped in cohorts according to lifetimes. Note that removal of the PTEN-like domain but not the J domain largely inhibited the burst-like recruitment of Aux1. The numbers of analysed traces are shown above each cohort. Data are representative of at least two independent experiments. b, Genomic PCR analysis showing biallelic integration of the TagRFP sequence into the CLTA genomic locus in the SUM159 clonal gene-edited CLTA–TagRFP+/+ cell line (top), representative of three independent experiments. Transferrin uptake comparing parental, unedited SUM159 control cells and the gene-edited CTLA–TagRFP+/+ cells determined by flow cytometry (n = 3 independent experiments, mean ± s.d.) (bottom). c–e, Schematic representation of the constructs EGFP–Aux1, EGFP–Aux1(420–814) and EGFP–Aux1(547–814) used for transient expression in gene-edited AP2–TagRFP+/+ SUM159 cells and then imaged by TIRF microscopy. The plots show averaged fluorescence intensity traces (mean ± s.e.m.) of AP2–TagRFP+/+ (red) and of the Aux1 constructs (green) from endocytic clathrin-coated pits and vesicles identified in 5, 6 and 6 cells, respectively, and then grouped in cohorts according to lifetimes. Note that removal of residues 420–546 from EGFP–Aux1(420–814) has largely inhibited its small, burst-like recruitment. The numbers of analysed traces are shown above each cohort. Data are representative of two independent experiments. f, A linker derived from the unstructured region (residues 508–736) of Dishevelled2 (Dvl2) was inserted between EGFP and Aux1(547–814) to make the construct EGFP–Dvl2(508–736)–Aux1(547–814), which showed very small burst-like recruitment, similar to that of EGFP–Aux1(420–814), which also lacked the PTEN-like domain (n = 5 cells). Data are representative of two independent experiments. g, h, The constructs EGFP–PH(PLCδ1)-Aux1(420–814) or EGFP–PH(PLCδ1)-Aux1(547–814) were used for transient expression in AP2–TagRFP+/+ cells and then imaged by TIRF microscopy. The plots show averaged fluorescence intensity traces (mean ± s.e.m.) of AP2–TagRFP+/+ (red) and of the Aux1-based PtdIns(4,5)P2 sensors (green) associated with endocytic clathrin-coated pits and vesicles identified in 9 and 6 cells, and then grouped in cohorts according to their lifetimes. Note that the removal of the linker Aux1 residues 420–546 from EGFP–PH(PLCδ1)-Aux1(420–814) largely inhibited its recruitment to coated pits (h). The numbers of analysed traces are shown above each cohort. Data are representative of two independent experiments. i, The Dvl2(508–736) linker was inserted between EGFP–PH(PLCδ1) and Aux1(547–814) to make the PtdIns(4,5)P2 sensor EGFP–PH(PLCδ1)–Dvl2(508–736)–Aux1(547–814). This chimaera is recruited to coated pits with an efficiency similar to that of EGFP–PH(PLCδ1)-Aux1(420–814) (n = 12 cells). Data are representative of two independent experiments. j, Table summarizing general phosphoinositide probes and Aux1-based phosphoinositide sensors used in this study. The results obtained with sensors specific for PtdIns3P, PtdIns4P, PtdIns(4,5)P2, and PtdIns(3,4)P2, were obtained using the tandem FYVE domains of Hrs, the P4M domain of DrrA, the PH domain of PLCδ1 and the tandem PH domains of TAPP1, respectively.
Extended Data Figure 2 Restricted localization and lipid specificity of the Aux1-based phosphoinositide sensors.
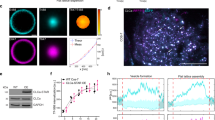
a, Intracellular distribution of the Aux1-based PtdIns(4,5)P2 sensor recorded by lattice light-sheet microscopy. The representative images are from a CLTA–TagRFP+/+ (red) cell expressing the Aux1-based PtdIns(4,5)P2 sensor EGFP–PH(PLCδ1)-Aux1. The left panel shows a deconvolved 3D rendering from a single time point and illustrates the location of the optical section shown in the right panels. The images contrast the full colocalization throughout the plasma membrane of the fluorescent signals corresponding to the sensor and clathrin with the absence of colocalization in endosomal clathrin-containing structures. The EGFP channel in the right lower panel was shifted laterally by six pixels. b, Bottom surface of a gene-edited AP2–EGFP+/+ cell, bearing endocytic AP2 complexes labelled by σ2–EGFP and co-expressing CIBN–CAAX and the mCherry–tagged inositol 5-phosphatase module of OCRL (mCherry–CRY2-5-ptaseOCRL), imaged at 1-s intervals for 200 s by spinning-disk confocal microscopy. Acute depletion of plasma membrane PtdIns(4,5)P2, by recruitment of mCherry–CRY2-5-ptaseOCRL from the cytosol to the plasma membrane, was triggered by illumination with 488-nm light beginning at t = 0 s. The kymograph shows that partially formed coated pits stalled and new ones failed to initiate owing to loss of PtdIns(4,5)P2. c, Bottom surface of a SUM159 cell co-expressing the PtdIns(4,5)P2 sensor EGFP–PH(PLCδ1)-Aux1 together with CIBN–CAAX and mCherry–CRY2-5-ptaseOCRL imaged at 2-s intervals for 100 s by spinning-disk confocal microscopy. Acute, light-mediated depletion of plasma membrane PtdIns(4,5)P2 was initiated at t = 0 s. The kymograph illustrates loss of membrane recruitment of the Aux1-based PtdIns(4,5)P2 sensor (top). Recruitment of the Aux1-based PtdIns(4,5)P2 sensor to assembling endocytic coated pits was not affected when a mutant of the phosphatase module of OCRL, unable to hydrolyse PtdIns(4,5)P2, was targeted to the plasma membrane by light activation (bottom). SUM159 cells co-expressing the PtdIns(4,5)P2 sensor EGFP–PH(PLCδ1)-Aux1, together with CIBN–CAAX and the catalytically inactive mCherry–CRY2-5-ptaseOCRL(D523G) (bottom). Images at single time points and the corresponding kymograph are shown. d, Early recruitment of the Aux1-based PtdIns4P sensor to stalled coated pits was not affected when the active phosphatase module of OCRL was targeted to the plasma membrane by light activation (top). The late burst of the PtdIns4P sensor was absent, however, consistent with failure of the stalled (that is, persistent) coated pits to finish assembly and hence failure to bud into coated vesicles. As expected, membrane targeting of the mutant phosphatase module of OCRL had no effect on recruitment of the PtdIns4P sensor to coated pits and vesicles (bottom). The cells co-expressed the PtdIns4P sensor EGFP–P4M(DrrA)-Aux1, together with CIBN–CAAX and mCherry–CRY2-5-ptaseOCRL (top) or the catalytically inactive mCherry–CRY2-5-ptaseOCRL(D523G) (bottom). The bottom surface of the cells was imaged using spinning-disk confocal microscopy. e, Blocking dynamin activity prevented the acute burst but not the binding of the PtdIns4P sensor to arrested (that is, persistent) coated structures at the plasma membrane. AP2–TagRFP+/+ cells expressing the PtdIns4P sensor EGFP-P4M(DrrA)-Aux1 were treated for 10 min without or with dynasore-OH (30 μM) and then imaged using TIRF microscopy. f, CLTA-TagRFP+/+ cells stably expressing the PtdIns4P sensor EGFP–P4M(DrrA)-Aux1 were treated with siRNA targeting dynamin2 (DNM2) or with a control sequence, and imaged by TIRF microscopy. The kymograph from a time series of the cell depleted of dynamin2 shows stalling of coated pits, absence of acute PtdIns4P burst and maintenance of the binding of the PtdIns4P sensor to arrested (that is, persistent) coated structures at the plasma membrane. The right panel shows the efficiency of DNM2 mRNA depletion measured by real-time quantitative PCR (n = 3 independent experiments, mean ± s.d.). g, Direct comparison of the recruitment dynamics at endocytic clathrin-coated structures of the Aux1-based PtdIns(4,5)P2 and PtdIns4P sensors. The left panels show a representative image and the corresponding kymograph from a time series, obtained by TIRF microscopy from the bottom surface of a SUM159 cell co-expressing the PtdIns(4,5)P2 sensor (green) and the PtdIns4P sensor (red). The central panel shows the averaged fluorescence intensity traces (mean ± s.e.m.) of the PtdIns(4,5)P2 sensor (green) and the PtdIns4P sensor (red), including the numbers of traces analysed for each lifetime cohort. The right panel is a schematic representation of the early (Early) and late (Burst) recruitment of the PtdIns4P sensor during clathrin-coated pit formation. Note that although the PtdIns4P and PtdIns(4,5)P2 sensors are both recruited early during coated pit formation, only the PtdIns4P sensor then appears as a transient burst coinciding with decline of the PtdIns(4,5)P2 signal. The EGFP channel in all the kymographs with EGFP and TagRFP overlaid was shifted laterally by six pixels. Data are representative of at least two independent experiments. Scale bars, 5 μm.
Extended Data Figure 3 Validation of the Aux1-based phosphoinositide-binding sensors.
a, The lipid-binding regions in the Aux1-based sensors are required for their phosphoinositide specificity. Shown are representative chemiluminescence images obtained from the lipid–protein overlay assay using the indicated Aux1-based sensors. The strip contained lysophosphatidic acid (LPA), lysophosphocholine (LPC), PtdIns, PtdIns3P, PtdIns4P, PtdIns5P, phosphatidylethanolamine (PE), phosphatidylcholine (PC), sphingosine-1-phosphate (S1P), PtdIns(3,4)P2, PtdIns(3,5)P2, PtdIns(4,5)P2, PtdIns(3,4,5)P3, phosphatidic acid (PA), and phosphatidylserine (PS). The lipids that recruited the various sensors are highlighted in red. b, The relatively low levels of transient expression of the Aux1-based phosphoinositide sensors, as used in this study, did not detectably affect the assembly dynamics of endocytic clathrin-coated pits and vesicles. The plot shows the distributions of lifetimes and number of clathrin traces determined by 2D automated analysis obtained from time series acquired by TIRF microscopy. The data are from SUM159 cells gene-edited for CLTA–TagRFP+/+ and EGFP–Aux1+/+, or gene-edited for CLTA–TagRFP+/+ and transiently expressing EGFP–Aux1, the PtdIns(4,5)P2 sensor EGFP–PH(PLCδ1)-Aux1, the PtdIns3P sensor EGFP–2×FYVE(Hrs)-Aux1, the PtdIns4P sensor EGFP–P4M(DrrA)-Aux1, or the PtdIns(3,4)P2 sensor EGFP–2×PH(TAPP1)-Aux1. There was no significant difference between the distributions of coated pit lifetimes between cells expressing the sensors and the double gene-edited EGFP–Aux1+/+ and CLTA–TagRFP+/+ cells used as control. Data are mean ± s.d. Cohen’s d values with 95% confidence interval (CI) are: 0.03 [−0.01, 0.08], 0.04 [0, 0.09], 0.07 [0.02, 0.11], 0.11 [0.07, 0.15], 0.01 [−0.03, 0.05]. c, The expression of the Aux1-based sensors do not affect the receptor-mediated uptake of transferrin. The plots show the absence of effect in the extent of internalized (top) and membrane-bound (bottom) Alexa Fluor 647-conjugated transferrin in the control non-transfected SUM159 cells and SUM159 cells stably expressing increasing amounts of the various Aux1-based EGFP-tagged sensors as determined by flow cytometry. Data are representative of two independent experiments.
Extended Data Figure 4 Expression of the Aux1-based sensors in SUM159 cells, COS-7 cells and human fibroblasts, and expression of the chimaeric sensors containing the epsin1 clathrin-binding domain in SUM159 cells.
a, Bottom surfaces of CLTA–TagRFP+/+ cells transiently expressing EGFP-tagged sensors specific for PtdIns(4,5)P2, PtdIns3P, PtdIns4P and PtdIns(3,4)P2, imaged by TIRF microscopy for 300 s at 1-s intervals. Each set of results shows data obtained from a wild-type Aux1-based sensor specific to the corresponding phosphoinositide (left), an Aux1-based sensor mutated to eliminate phosphoinositide binding (middle) and a general sensor with defined phosphoinositide specificity (right). The representative images correspond to a single time point. b, Gene-edited CLTA–TagRFP+/+ SUM159 cells stably expressing the indicated Aux1-based phosphoinositide sensors were imaged by TIRF microscopy. The plots show average fluorescence intensity traces (mean ± s.e.m.) of TagRFP–CLTA (red) and the phosphoinositide sensors (green) tracked in 17, 10, 12 and 14 cells and then grouped by cohorts according to their lifetimes. The numbers of analysed traces are shown above each cohort. c, COS-7 cells stably expressing TagRFP–CLTA and the indicated Aux1-based phosphoinositide sensors were imaged by TIRF microscopy and the traces from 7, 10, 9 and 18 cells were analysed as described in b. d, Human fibroblasts stably expressing TagRFP–CLTA and transiently expressing the Aux1-based phosphoinositide sensors were imaged by TIRF microscopy and the traces from 14, 11, 23 and 21 cells were analysed as described in b. e, The EGFP-tagged full-length epsin1, its EGFP-tagged clathrin-binding domain (residues 230–475), or EGFP-tagged sensors containing the indicated protein domains specific for defined phosphoinositides fused at their C terminus to the epsin1 clathrin-binding domain were transiently expressed in CLTA–TagRFP+/+ SUM159 cells and imaged by TIRF microscopy. The EGFP channel in the kymographs was shifted laterally by six pixels. The plots show averaged fluorescence intensity traces (mean ± s.e.m.) of CLTA–TagRFP (red) and the epsin1-based sensors (green) identified in 7, 8, 13, 11, 15 and 14 cells and then grouped by cohorts according to lifetimes. The numbers of analysed traces are shown above each cohort. Data are representative of at least two independent experiments. Scale bars, 5 μm.
Extended Data Figure 5 Association of the Aux1-based PtdIns(3,4)P2 sensor together with a few copies of clathrin with uncoated vesicles.
a, Blocking by the dynamin inhibitor dynasore-OH of accumulation of the PtdIns(3,4)P2 sensor on clathrin-coated structures in AP2–TagRFP+/+ cells transiently expressing the PtdIns(3,4)P2 sensor EGFP–2×PH(TAPP1)-Aux1 (top). The cells were incubated for 10 min without or with 30 μM dynasore-OH and then imaged by spinning-disk confocal microscopy at the bottom surface. The representative kymograph highlights the expected stalling of coated pits induced by brief exposure to dynasore-OH together with their failure to recruit the PtdIns(3,4)P2 sensor. CLTA–TagRFP+/+ cells stably expressing the PtdIns(3,4)P2 sensor EGFP–2×PH(TAPP1)-Aux1 were treated with siRNA targeting dynamin2 or SNX9, or with a control sequence, and imaged by TIRF microscopy (bottom). The kymograph from a time series of a cell depleted of dynamin2 shows that the stalled (that is, persistent) coated pits lack the PtdIns(3,4)P2 sensor. The bottom panels show the efficiency of depletion of SNX9 mRNA measured by real-time quantitative PCR (n = 3 independent experiments, mean ± s.d.) and the distributions of lifetimes for clathrin-coated structures in control (1,513 traces from 8 cells) and SNX9-KD cells (1,038 traces from 11 cells). b, Representative fluorescence traces obtained from gene-edited dynamin2–EGFP+/+ SUM159 cells transiently expressing the Aux1-based PtdIns(3,4)P2 sensor mCherry–2×PH(TAPP1)-Aux1 imaged by TIRF microscopy. Recruitment of the PtdIns(3,4)P2 sensor follows loss of the dynamin signal corresponding to the membrane scission associated with coated pit budding and formation of a coated vesicle. c, Temporal relationship between recruitment of the Aux1-based PtdIns(3,4)P2 sensor and the onset of uncoating of endocytic clathrin-coated vesicles. CLTA–TagRFP+/+ cells transiently expressing the PtdIns(3,4)P2 sensor EGFP–2×PH(TAPP1)-Aux1 were imaged by spinning-disk confocal microscopy towards the leading edge of their bottom surface. Tracking endocytic events near the leading edge of the cell where the uncoated vesicles show limited vertical movement enabled us to capture examples of endocytic events still containing a small number of clathrin triskelions associated with uncoated vesicles (arrows). The representative kymographs (left) and corresponding fluorescence intensity traces (right) from naked vesicles show association of the PtdIns(3,4)P2 sensor together with a few copies of clathrin (arrow) after culmination of the uncoating process. d, Double gene-edited CLTA–TagRFP+/+ and EGFP–Rab5c+/+ SUM159 cells were imaged by TIRF microscopy at the leading edge of their bottom surface. Representative kymographs (left) and corresponding fluorescence intensity traces (right) highlight that a small number of clathrin triskelions remained associated with the uncoated vesicles (arrows). Temporal relationship showing that onset of Rab5c recruitment occurred after the uncoating of endocytic clathrin-coated vesicles. The EGFP channel in all the kymographs with EGFP and TagRFP overlaid was shifted laterally by six pixels. Data are representative of at least two independent experiments. Scale bars, 5 μm.
Extended Data Figure 6 Effects of interfering with the activity of inositol kinases and inositol phosphatases on the PtdIns4P and PtdIns(4,5)P2 content of endocytic clathrin-coated structures.
a, Inhibition of PI4KIIIα by the small molecule A1 increased the number of longer-lived coated pits. CLTA–TagRFP+/+ cells stably expressing the PtdIns4P sensor EGFP–P4M(DrrA)-Aux1 were treated for 10 min with DMSO alone, or with DMSO and 100 nM A1, and then imaged by TIRF microscopy. The distributions of coated-structure lifetimes for the control (1,413 traces from 5 cells) and for A1 treated cells (864 traces from 14 cells) are shown. b, Inhibition of PI4KIIIα by A1 is reversible. CLTA–TagRFP+/+ cells expressing the PtdIns4P sensor EGFP–P4M(DrrA)-Aux1 were treated for 10 min with DMSO alone, or with DMSO and 100 nM A1, and then imaged by TIRF microscopy. To verify reversibility, the medium containing A1 was removed for 30 min and the cells also imaged by TIRF microscopy. c, Representative kymographs from a time series obtained by TIRF microscopy comparing recruitment of the PtdIns(4,5)P2 sensor EGFP–PH(PLCδ1)-Aux1 stably expressed in control CLTA–TagRFP+/+ cells and cells treated with the inhibitor A1. The PtdIns(4,5)P2 sensor was still recruited in the presence of the A1 inhibitor. d, The left panel shows efficient depletion of PIP5K1C mRNA measured by real-time quantitative PCR in CLTA–TagRFP+/+ cells stably expressing the PtdIns(4,5)P2 sensor EGFP–PH(PLCδ1)-Aux1 (n = 3 independent experiments). The plots show averaged fluorescence intensity traces of the PtdIns(4,5)P2 sensor associated with endocytic clathrin-coated pits identified in 9 control and 11 PIPKIγ-KD cells, grouped in cohorts according to lifetimes. The numbers of analysed traces are shown above each cohort. Data are from time series obtained by TIRF microscopy. e, Genomic PCR analysis showing biallelic integration of the EGFP sequence into the SYNJ1 genomic locus in two clonal gene-edited SUM159 cell lines expressing EGFP–Synj1+/+ and CTLA–TagRFP+/+. Clone A1 was used in this study. f, The representative kymograph from a time series acquired by TIRF microscopy of SUM159 cells double gene-edited for EGFP–Synj1+/+ and CLTA–TagRFP+/+. Representative traces show the fluorescence intensity profile of CLTA–TagRFP (red) and the number of EGFP–Synj1 molecules (green) associated with two endocytic events. Synj1 was recruited erratically throughout all stages of clathrin-coated pit formation. g, Knockout of Synj1 in gene-edited CLTA–TagRFP+/+ SUM159 cells by CRISPR–Cas9 targeted knockout of SYNJ1. There were minor differences between wild-type and knockout cells in the fluorescence intensities of the recruited PtdIns4P sensor during the early (Cohen’s d = 0.29) and late burst stages (Cohen’s d = 0.12), comparing 1,388 and 1,150 traces from 16 control and 10 knockout cells, respectively. h, Knockdown of Synj1 in gene-edited CLTA–TagRFP+/+ SUM159 cells stably expressing the PtdIns4P sensor EGFP–P4M(DrrA)-Aux1. The left panel shows the efficiency, measured by real-time quantitative PCR, of SYNJ1 mRNA knockdown mediated by lentivirus transduction with shRNA (n = 5 independent experiments). Data in the central and right panels are from time series obtained by TIRF microscopy. There were no significant differences in the fluorescence intensity of the recruited PtdIns4P sensor from the early (Cohen’s d = 0.02) or late burst stages (Cohen’s d = 0.24), comparing 760 and 1,499 traces from 7 control and 9 knockdown cells, respectively. i, Knockdown of Synj1 in COS-7 cells stably expressing TagRFP–CLTA and the PtdIns4P sensor EGFP–P4M(DrrA)-Aux1. The left panel shows the efficiency, measured by real-time quantitative PCR, of SYNJ1 mRNA knockdown mediated by lentivirus transduction with shRNA (n = 3 independent experiments). Data in the central and right panels are from time series obtained by TIRF microscopy. There were no significant differences in the fluorescence intensity of the recruited PtdIns4P sensor from the early (Cohen’s d = 0.05) and late burst stages (Cohen’s d = 0.04), comparing 1,430 and 1,051 traces from 26 control and 25 knockdown cells, respectively. Data are mean ± s.d. in d (bar graph), g–i and mean ± s.e.m. in d (cohorts). Data are representative of at least two independent experiments. Cohen’s d with 95% CI are: [0.21, 0.37] and [0.04, 0.20] (g); [−0.07, 0.11] and [0.15, 0.33] (h); [−0.03, 0.12] and [−0.04, 0.12] (i). The EGFP channel in all the kymographs was shifted laterally by six pixels. Scale bars, 5 μm.
Extended Data Figure 7 Effect of interfering, individually or jointly, with the activities of inositol 5-phosphatases on the PtdIns4P and PtdIns(4,5)P2 content of endocytic clathrin-coated structures.
a, Genomic PCR analysis showing biallelic integration of the EGFP sequence into the OCRL genomic locus in the clonal gene-edited EGFP–OCRL+/+ SUM159 cell line. b, Representative kymograph from a time series obtained by TIRF microscopy of EGFP–OCRL+/+ cells transiently expressing mCherry–CLTA, showing the burst-like recruitment of EGFP–OCRL during vesicle uncoating. The EGFP channel was shifted laterally by six pixels. Scale bar, 5 μm. c, Distribution of the maximum number of EGFP–OCRL molecules recruited during uncoating to individual clathrin-coated vesicles in EGFP–OCRL+/+ cells (606 traces from 12 cells). d, Representative plot of one endocytic event showing fluorescence intensity traces of EGFP–OCRL (expressed as number of recruited molecules) and the PtdIns4P sensor mCherry–P4M(DrrA)-Aux1 from a time series obtained by TIRF microscopy of double gene-edited EGFP–OCRL+/+ cells expressing the PtdIns4P sensor. e, Expression of OCRL was eliminated in CLTA–TagRFP+/+ cells by CRISPR–Cas9 targeted knockout of OCRL as determined by western-blot analysis using antibodies against OCRL and actin (loading control). The fluorescence intensities of the recruited PtdIns4P sensor were obtained by comparing 1,177 and 1,015 traces from 10 control and 11 knockout cells, respectively. f, Reduction in the expression of OCRL mediated by siRNA in CLTA–TagRFP+/+ cells was confirmed by western blot analysis using antibodies against OCRL and actin. CLTA–TagRFP+/+ cells stably expressing the PtdIns4P sensor EGFP–P4M(DrrA)-Aux1 were treated with siRNA targeting either OCRL or a control sequence, and imaged by TIRF microscopy. No significant differences were observed in the fluorescence intensity associated with the recruitment of the PtdIns4P sensor during the early (Cohen’s d = 0.04) or late burst stages (Cohen’s d = 0.20) in 740 and 1,442 traces from 6 control and 5 knockdown cells, respectively. g, Knockdown of OCRL in COS-7 cells stably expressing TagRFP–CLTA and the PtdIns4P sensor EGFP–P4M(DrrA)-Aux1. Efficiency of OCRL depletion by siRNA was determined by real-time quantitative PCR (n = 3 independent experiments). PtdIns4P sensor recruitment data obtained from time series obtained by TIRF microscopy showed there were no significant differences in the fluorescence intensity of the recruited PtdIns4P sensor from the early (Cohen’s d = 0.09) and late burst stages (Cohen’s d = 0.09), comparing 709 and 848 traces from 15 control and 17 knockdown cells, respectively. h, Elimination of Synj1 together with approximately 80% depletion of OCRL (Synj1-KO + OCRL-KD) increased the recruitment of the PtdIns(4,5)P2 sensor to coated pits and vesicles. Expression was reduced by CRISPR–Cas9 targeted knockout of OCRL or Synj1 and by siRNA of OCRL in CLTA–TagRFP+/+ cells transiently expressing the PtdIns(4,5)P2 sensor EGFP–PH(PLCδ1)-Aux1. The cells were imaged by TIRF microscopy, and the plots show the maximum fluorescence intensity of the PtdIns(4,5)P2 sensor recruited at the approximate time of vesicle budding (1,436, 843, 2,142, 897 and 1,674 traces) and the lifetimes of the coated vesicles (1,851, 1,320, 2,534, 1,076 and 1,876 traces) from 10 control, 9 OCRL-KO, 11 OCRL-KD, 11 Synj1-KO and 11 Synj1-KO + OCRL-KD cells, respectively. The recruitment of the PtdIns(4,5)P2 sensor in Synj1-KO + OCRL-KD cells was significantly higher than control cells (Cohen’s d = 0.53). i, Elimination of Synj1 together with approximately 80% depletion of OCRL (Synj1-KO + OCRL-KD) did not affect recruitment of the PtdIns3P sensor (Cohen’s d = 0.17) to coated vesicles, comparing 918 and 990 traces from 14 control and 15 Synj1-KO + OCRL-KD cells, respectively. j, The expression levels of mRNA for OCRL and SYNJ1, measured by real time quantitative PCR, in control, OCRL-KD and Synj1-KD cells (n = 4 experiments for OCRL-KD and n = 3 experiments for Synj1-KD). Data are mean ± s.d. Data are representative of at least two independent experiments. Cohen’s d with 95% CI are: [−0.05, 0.12] and [0.11, 0.28] (f); [−0.01, 0.19] and [−0.01, 0.19] (g); [0.46, 0.60] (h); [0.08, 0.26] (i).
Extended Data Figure 8 No changes in the PtdIns(3,4)P2 content of endocytic clathrin-coated structures induced by interference with the activity of candidate inositol kinases.
a, Genomic PCR analysis (left) showing single allelic integration of the EGFP sequence into the PIK3C2A genomic locus in the clonal gene-edited EGFP–PI3K-C2α+/− SUM159 cells. Western blot analysis (right) of cell lysates probed with antibodies against PI3K-C2α and actin from SUM159 cells and EGFP–PI3K-C2α+/− cells show expression of EGFP–PI3K-C2α. b, Dual gene-edited EGFP–PI3K-C2α+/− and CLTA–TagRFP+/+ SUM159 cells were imaged by TIRF microscopy. The plots show averaged fluorescence intensity traces (mean ± s.e.m.) of CLTA–TagRFP and EGFP–PI3K-C2α corresponding to the cohort of coated pits lasting 40–60 s (237 traces from 6 cells). c, Representative plot from a time series obtained by TIRF microscopy of a EGFP–PI3K-C2α+/− cell transiently expressing the PtdIns3P sensor mCherry–2×FYVE(Hrs)-Aux1. The tracing highlights the presence of a few copies of EGFP–PI3K-C2α at the time the PtdIns3P burst was detected. d, CLTA–TagRFP+/+ cells transiently expressing EGFP–PI3K-C2α were imaged by TIRF microscopy. The plots show averaged fluorescence intensity traces (mean ± s.e.m.) of CLTA–TagRFP and EGFP–PI3K-C2α corresponding to the cohort of coated pits lasting 40–60 s (697 traces from 10 cells). e, Reduction in the expression of PI3K-C2α following treatment with siRNA was confirmed by western blot analysis using antibodies against PI3K-C2α and actin. f, PI3K-C2α is partially required for the burst recruitment of the PtdIns3P sensor. SUM159 cells stably expressing the PtdIns3P sensor EGFP–2×FYVE(Hrs)-Aux1 were treated with control siRNA (1) or with siRNA specific for PI3K-C2α (2) to transiently deplete its expression. Cells treated with siRNA for PI3K-C2α were also transfected with either siRNA-resistant wild-type mCherry–PI3K-C2α (3) or kinase-deficient mCherry–kdPI3K-C2α (4) one day before TIRF imaging. Burst recruitment of the PtdIns3P sensor was prevented by depletion of PI3K-C2α (Cohen’s d = 0.52, comparing 2,150 and 674 traces from 10 control cells and 10 PI3K-C2α-KD cells, respectively) and was only rescued upon expression of wild-type mCherry–PI3K-C2α (Cohen’s d = 0.72, comparing 3,304 and 674 traces from 9 wild-type PI3K-C2α-expressing cells and 10 PI3K-C2α-KD cells, respectively) but not the kinase-deficient mCherry–kdPI3K-C2α (Cohen’s d = 0.11, comparing 1,113 and 674 traces from 12 kinase-deficient PI3K-C2α-expressing cells and 10 PI3K-C2α-KD cells, respectively). g, CLTA–TagRFP+/+ cells stably expressing the PtdIns(3,4)P2 sensor EGFP–2×PH(TAPP1)-Aux1 were treated with control siRNA or with siRNA specific for PI3K-C2α to transiently deplete its expression. Data analysis from time series obtained by TIRF microscopy showed that PI3K-C2α depletion did not affect the maximum fluorescence intensity of the recruited PtdIns(3,4)P2 sensor (Cohen’s d = 0.05) determined for 1,136 and 1,579 traces from 10 control and 10 PI3K-C2α-KD cells, respectively. h, CLTA–TagRFP+/+ cells stably expressing the PtdIns4P sensor EGFP–P4M(DrrA)-Aux1 were treated with siRNA to knockdown the expression of PI3K-C2α, and time series were obtained by TIRF microscopy. PI3K-C2α depletion did not affect the fluorescence intensity of the recruited PtdIns4P sensor during the early (Cohen’s d = 0.07) or late burst stages (Cohen’s d = 0.09), determined for 1,394 and 2,316 traces from 11 control cells and 12 PI3K-C2α-KD cells, respectively. i, PI3K-C2α is partially required for the burst recruitment of the PtdIns3P sensor in COS-7 cells stably expressing TagRFP–CLTA together with the PtdIns3P sensor EGFP–2×FYVE(Hrs)-Aux1 or the PtdIns(3,4)P2 sensor EGFP–2×PH(TAPP1)-Aux1. The efficiency of PI3K-C2α depletion was determined by real-time quantitative PCR (left, n = 4 independent experiments). Analysis of time series obtained by TIRF microscopy shows a significant decrease in the recruitment of the PtdIns3P sensor (middle, Cohen’s d = 0.82) in cells depleted of PI3K-C2α (569 traces from 16 cells) when compared with cells treated with control siRNA (787 traces from 12 cells). PI3K-C2α depletion did not affect the maximum fluorescence intensity of the recruited PtdIns(3,4)P2 sensor (right, Cohen’s d = 0.10) determined for 1,229 and 1,158 traces from 24 control and 27 PI3K-C2α-KD cells, respectively. j, Inhibition of Vps34 by the small molecule VPS34-IN1. CLTA–TagRFP+/+ cells stably expressing the PtdIns3P sensor EGFP–2×FYVE(Hrs)-Aux1 were treated for 1 h with 5 μM VPS34-IN1 and time series obtained by TIRF microscopy. Inhibition of Vps34 did not affect the maximum fluorescence intensity of the recruited PtdIns3P sensor during the late burst stage of recruitment (Cohen’s d = 0.01), comparing 853 and 522 traces from 9 control DMSO and 12 VPS34-IN1 treated cells, respectively. k, INPP4A is partially required for the burst recruitment of the PtdIns3P sensor. Efficiency of INPP4A depletion by siRNA in CLTA–TagRFP+/+ cells stably expressing the PtdIns3P sensor EGFP–2×FYVE(Hrs)-Aux1 or the PtdIns(3,4)P2 sensor EGFP–2×PH(TAPP1)-Aux1 was determined by real-time quantitative PCR (left, n = 3 independent experiments, mean ± s.d.). Analysis of time series obtained by TIRF microscopy (middle) shows a significant decrease in the recruitment of the PtdIns3P sensor (Cohen’s d = 0.64) in cells depleted of INPP4A (511 traces from 12 cells) when compared with cells treated with control siRNA (1,179 traces from 9 cells). INPP4A depletion has a minor effect on the recruitment of the PtdIns(3,4)P2 sensor (Cohen’s d = 0.23) determined for 866 and 274 traces from 13 control and 14 INPP4A-KD cells, respectively. INPP4A-depleted cells (n = 12) had significantly more arrested pits than control cells (n = 9) (right, P = 0.0002, unpaired two-tailed Student’s t-test). Data are mean ± s.e.m. in b, d and mean ± s.d. in f–k. Data are representative of at least two independent experiments. Cohen’s d with 95% CI are: [0.43, 0.61], [0.64, 0.81] and [0.02, 0.21] (f); [−0.03, 0.12] (g); [0.00, 0.13] and [0.02, 0.15] (h); [0.71, 0.93] and [0.02, 0.18] (i); [−0.10, 0.12] (j); [0.53, 0.74] and [0.09, 0.37] (k).
Extended Data Figure 9 Recruitment of Rab5 to clathrin-derived endocytic carriers.
a, Genomic PCR analysis showing biallelic integration of the EGFP sequence into the RAB5A genomic locus in gene-edited EGFP–Rab5a+/+ SUM159 cells (left), into the RAB5A and RAB5C genomic loci of EGFP–Rab5a+/+ EGFP–Rab5c+/+ SUM159 cells (middle), and into the RAB5C genomic locus of CLTA–TagRFP+/+ EGFP–Rab5c+/+ SUM159 cells (right), respectively. b, Rab5a was not recruited to clathrin-coated pits or vesicles; the onset of recruitment followed conclusion of the uncoating stage of endocytic clathrin-coated vesicles (events 1 and 4). EGFP–Rab5a+/+ cells transiently expressing mCherry–CLTA were imaged by TIRF microscopy. The representative kymograph and fluorescence intensity traces including the number of recruited Rab5a molecules are shown for four endocytic events. c, EGFP–Rab5a+/+ cells transiently expressing mRFP–EEA1 were imaged at the middle plane by spinning-disk confocal microscopy. d, Neither Rab5a nor Rab5c in gene-edited cells were recruited to clathrin-coated pits or vesicles; the onset of recruitment followed conclusion of the uncoating stage of endocytic clathrin-coated vesicles. EGFP–Rab5a+/+ and EGFP–Rab5c+/+ cells transiently expressing mCherry–CLTA were imaged by TIRF microscopy. The representative kymograph and fluorescence intensity traces including the number of recruited Rab5a and Rab5c molecules for three endocytic events are shown. e, Rab5c was not recruited to clathrin-coated pits or vesicles in gene-edited SVGA EGFP–Rab5c+/+ cells transiently expressing mCherry–CLTA. The cells were imaged by TIRF microscopy. A representative kymograph and fluorescence intensity traces for one endocytic event (arrow) are shown. f, Transiently expressed EGFP–Rab5a was not recruited to clathrin-coated pits or vesicles in CLTA–TagRFP+/+ SUM159 cells. Cells were imaged by TIRF microscopy. The representative kymograph and fluorescence intensity traces including the number of recruited Rab5a molecules for one endocytic event (arrow) are shown. The EGFP channel in all the kymographs with EGFP and mCherry or TagRFP overlaid was shifted laterally by six pixels. Data are representative of two independent experiments. Scale bars, 5 μm.
Extended Data Figure 10 Endocytic clathrin-coated vesicles acquire Rab5 before fusing with endosomes.
a, EGFP–Rab5c+/+ SUM159 cells transiently expressing the Aux1-based PtdIns(3,4)P2 sensor mCherry–2×PH(TAPP1)-Aux1 were imaged in 3D by lattice light-sheet microscopy (time series 300 s in duration, where each time point consisted of a stack of 41 planes spaced approximately 261 nm apart imaged with an approximately 2.5-s interval between stacks). The 3D plot (left) shows the 3D position of every one of the tracked PtdIns(3,4)P2-containing objects colour-coded from red to green (colour bar) as the linear ratio of recruited PtdIns(3,4)P2 sensor with respect to the amount of Rab5c content changed over time. The 3D data are from 1,265 traces detected in 16 cells from 2 independent experiments. The directed movement of the traces starting at (0,0,0) becomes apparent when the capture of Rab5c becomes significant (left; Supplementary Video 7). The panel on the right shows the displacement of PtdIns(3,4)P2-containing objects versus the square root of time traces. The objects tracked at the top and bottom of the cell are shown in light blue and green, and their averages are shown in dark blue and green, respectively. The PtdIns(3,4)P2-containing objects tracked at the top and bottom of the cell (right) display a linear dependence of displacement with the square root of time consistent with non-directional 3D Brownian motion. The individual traces are depicted in light blue and green and the plots include the corresponding average displacements calculated for each time point (dark colour) and their fitted linear regressions (dashed lines with slopes of 0.19 ± 0.02 and 0.16 ± 0.02, respectively). b, Rab5 in endocytic carriers derived from clathrin-coated vesicles does not contain the early endosomal marker EEA1. EGFP–Rab5c+/+ SUM159 cells transiently expressing HaloTag–EEA1 and the PtdIns(3,4)P2 sensor mCherry–2×PH(TAPP1)-Aux1 were briefly incubated with the HaloTag ligand labelled with the Janelia Fluor 646 dye and then imaged at their bottom surface by spinning-disk confocal microscopy. The representative kymograph and fluorescence intensity traces for one endocytic event (arrow) are shown. Data are representative of two independent experiments. Scale bars, 5 μm. c, Depletion efficiency of the Rab5 GEFs hRME-6 or Rabex5 mRNA by siRNA in double gene-edited CLTA–TagRFP+/+ and EGFP–Rab5c+/+ SUM159 cells was determined by real-time quantitative PCR (upper left panel, n = 3 independent experiments, mean ± s.d.). The control or knockdown cells were imaged by TIRF microscopy. Rabex5 or hRME-6 depletion has a minor effect on the lifetime distributions of coated pits as determined from 1,097, 1,793, 1,829 and 1,782 traces (mean ± s.d.) imaged in 8 control, 11 Rabex5-KD, 10 hRME-6-KD and 11 Rabex5-KD + hRME-6-KD cells, respectively (upper right panel; Cohen’s d = 0.01, 0.11 and 0.15 with 95% CI [−0.06, 0.09], [0.03, 0.18] and [0.07, 0.22], respectively). The plots (lower panels) show averaged fluorescence intensity traces (mean ± s.e.m.) of EGFP–Rab5c (green) recruited during the uncoating stage of endocytic clathrin-coated vesicles (red) in control or knockdown cells. The numbers of analysed traces are shown above each cohort.
Supplementary information
Supplementary Information
This file contains a supplementary discussion and references. (PDF 398 kb)
Supplementary Data
This file contains supplementary figure 1 – uncropped gels. (PDF 1010 kb)
Recruitment dynamics of full-length Aux1 and Aux1 lacking its PTEN-like domain and the J-domain to endocytic clathrin-coated pits and vesicles.
Gene-edited CLTA-TagRFP+/+ SUM159 cells transiently expressing either full-length (EGFP-Aux1) or Aux1 lacking its PTEN-like and J-domains (EGFP-Aux1(420-814)) imaged at their bottom surfaces by spinning-disk confocal microscopy every 2 s during 5 min. To facilitate visualization, the EGFP channels in the right panels were shifted laterally by 6 pixels. (MP4 27999 kb)
Recruitment dynamics of the Aux1-based PtdIns(4,5)P2 sensor to endocytic clathrin-coated pits and vesicles.
Gene-edited CLTA-TagRFP+/+ SUM159 cells transiently expressing the Aux1-based PtdIns(4,5)P2 sensor EGFP-PH(PLCδ1)-Aux1 or the general PtdIns(4,5)P2 sensor EGFP-PH(PLCδ1) imaged at their bottom surfaces by spinning-disk confocal microscopy every 2 s during 5 min. To facilitate visualization, the EGFP channels in the right panels were shifted laterally by 6 pixels. (MP4 28357 kb)
Recruitment dynamics of the Aux1-based PtdIns3P sensor to clathrin-coated pits and vesicles.
Gene-edited CLTA-TagRFP+/+ SUM159 cell transiently expressing the Aux1-based PtdIns3P sensor EGFP-2xFYVE(Hrs)-Aux1 imaged at its bottom surface by TIRF microscopy every 1 s during 5 min. To facilitate visualization, the EGFP channel in the right panel was shifted laterally by 6 pixels. (MP4 28420 kb)
Recruitment dynamics of the Aux1-based PtdIns4P sensor to clathrin-coated pits and vesicles.
Gene-edited CLTA-TagRFP+/+ SUM159 cell transiently expressing the Aux1-based PtdIns4P sensor EGFP-P4M(DrrA)-Aux1 imaged at its bottom surface by TIRF microscopy every 1 s during 5 min. To facilitate visualization, the EGFP channel in the right panel was shifted laterally by 6 pixels. (MP4 27927 kb)
Recruitment dynamics of the Aux1-based PtdIns(3,4)P2 sensor to clathrin-coated vesicles and to uncoated vesicles.
Gene-edited CLTA-TagRFP+/+ SUM159 cell transiently expressing the Aux1-based PtdIns(3,4)P2 sensor EGFP-2xPH(TAPP1)-Aux1 imaged at its bottom surface by TIRF microscopy every 1 s during 5 min. To facilitate visualization, the EGFP channel in the right panel was shifted laterally by 6 pixels. (MP4 28700 kb)
Absence of Rab5 molecules in coated pits and coated vesicles.
Double gene-edited CLTA-TagRFP+/+ and EGFP-Rab5c+/+ SUM159 cell imaged at its bottom surface near the leading edge by TIRF microscopy every 1 s during 5 min. To facilitate visualization, the EGFP channel in the right panel was shifted laterally by 6 pixels. (MP4 29139 kb)
Recruitment of Rab5 to uncoated clathrin-derived endocytic carriers.
Gene-edited EGFP-Rab5c+/+ SUM159 cells transiently expressing the Aux1-based PtdIns(3,4)P2 sensor mCherry-2xPH(TAPP1)-Aux1 were imaged by lattice light-sheet microscopy in 3D (time series of 300 s in duration, where each time point was a stack of 41 planes spaced ~261 nm apart imaged at ~2.5 s intervals between stacks). The temporal changes in the three dimensional position and content of the fluorescent objects containing Rab5c and the PtdIns(3,4)P2 sensor were determined by automated 3D detection and tracking. The 3D time series plot shows the three dimensional position as a function of time of 1265 traces detected in 16 cells, color-coded from red to green as the ratio of the fluorescence signals of recruited PtdIns(3,4)P2 sensor with respect to Rab5c. a-c, The first frame of each of the aligned traces start at position (x,y,z) normalized to (0,0,0). Appearance of directed movement, coincident with a significant increase in the step-size of the displacement, followed capture of increased amounts of Rab5c; they are shown as single track (panel a), all tracks with a 5 frame rolling window (panel b), and cumulative trajectories (panel c). (AVI 2112 kb)
Rights and permissions
About this article
Cite this article
He, K., Marsland III, R., Upadhyayula, S. et al. Dynamics of phosphoinositide conversion in clathrin-mediated endocytic traffic. Nature 552, 410–414 (2017). https://doi.org/10.1038/nature25146
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature25146
This article is cited by
-
Adhesion energy controls lipid binding-mediated endocytosis
Nature Communications (2024)
-
Structure and dynamics of the EGFR/HER2 heterodimer
Cell Discovery (2023)
-
Long-term depression in neurons involves temporal and ultra-structural dynamics of phosphatidylinositol-4,5-bisphosphate relying on PIP5K, PTEN and PLC
Communications Biology (2023)
-
Phosphoinositides as membrane organizers
Nature Reviews Molecular Cell Biology (2022)
-
Structural basis of phosphatidylinositol 3-kinase C2α function
Nature Structural & Molecular Biology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.