Abstract
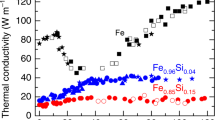
The Earth’s core is about ten per cent less dense than pure iron (Fe), suggesting that it contains light elements as well as iron. Modelling of core formation at high pressure (around 40–60 gigapascals) and high temperature (about 3,500 kelvin) in a deep magma ocean1,2,3,4,5 predicts that both silicon (Si) and oxygen (O) are among the impurities in the liquid outer core6,7,8,9. However, only the binary systems Fe–Si and Fe–O have been studied in detail at high pressures, and little is known about the compositional evolution of the Fe–Si–O ternary alloy under core conditions. Here we performed melting experiments on liquid Fe–Si–O alloy at core pressures in a laser-heated diamond-anvil cell. Our results demonstrate that the liquidus field of silicon dioxide (SiO2) is unexpectedly wide at the iron-rich portion of the Fe–Si–O ternary, such that an initial Fe–Si–O core crystallizes SiO2 as it cools. If crystallization proceeds on top of the core, the buoyancy released should have been more than sufficient to power core convection and a dynamo, in spite of high thermal conductivity10,11, from as early on as the Hadean eon12. SiO2 saturation also sets limits on silicon and oxygen concentrations in the present-day outer core.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Wood, B. J., Walter, M. J. & Wade, J. Accretion of the Earth and segregation of its core. Nature 441, 825–833 (2006)
Rubie, D. et al. Heterogeneous accretion, composition and core–mantle differentiation of the Earth. Earth Planet. Sci. Lett . 301, 31–42 (2011)
Righter, K. Prediction of metal–silicate partition coefficients for siderophile elements: an update and assessment of PT conditions for metal–silicate equilibrium during accretion of the Earth. Earth Planet. Sci. Lett . 304, 158–167 (2011)
Siebert, J., Badro, J., Antonangeli, D. & Ryerson, F. J. Terrestrial accretion under oxidizing conditions. Science 339, 1194–1197 (2013)
Bouhifd, M. A. & Jephcoat, A. P. Convergence of Ni and Co metal–silicate partition coefficients in the deep magma-ocean and coupled silicon–oxygen solubility in iron melts at high pressures. Earth Planet. Sci. Lett . 307, 341–348 (2011)
Takafuji, N., Hirose, K., Mitome, M. & Bando, Y. Solubilities of O and Si in liquid iron in equilibrium with (Mg,Fe)SiO3 perovskite and the light elements in the core. Geophys. Res. Lett . 32, L06313 (2005)
Siebert, J., Badro, J., Antonangeli, D. & Ryerson, F. J. Metal–silicate partitioning of Ni and Co in a deep magma ocean. Earth Planet. Sci. Lett . 321–322, 189–197 (2012)
Rubie, D. C. et al. Accretion and differentiation of the terrestrial planets with implications for the compositions of early-formed Solar System bodies and accretion of water. Icarus 248, 89–108 (2015)
Fischer, R. A. et al. High pressure metal–silicate partitioning of Ni, Co, V, Cr, Si, and O. Geochim. Cosmochim. Acta 167, 177–194 (2015)
Ohta, K., Kuwayama, Y., Hirose, K., Shimizu, K. & Ohishi, Y. Experimental determination of the electrical resistivity of iron at Earth’s core conditions. Nature 534, 95–98 (2016)
Konôpková, Z. et al. Direct measurement of thermal conductivity in solid iron at planetary core conditions. Nature 534, 99–101 (2016)
Tarduno, J. A., Cottrell, R. D., Davis, W. J., Nimmo, F. & Bono, R. K. A Hadean to Paleoarchean geodynamo recorded by single zircon crystals. Science 349, 521–524 (2015)
Sinmyo, R. & Hirose, K. The Soret diffusion in laser-heated diamond anvil cell. Phys. Earth Planet. Inter . 180, 172–178 (2010)
Helffrich, G. Outer core compositional layering and constraints on core liquid transport properties. Earth Planet. Sci. Lett . 391, 256–262 (2014)
Anzellini, S., Dewaele, A., Mezouar, M., Loubeyre, P. & Morard, G. Melting of iron at Earth’s inner core boundary based on fast x-ray diffraction. Science 340, 464–466 (2013)
Kato, C. et al. Melting in the FeO−SiO2 system to deep lower-mantle pressures: implications for subducted Banded Iron Formations. Earth Planet. Sci. Lett . 440, 56–61 (2016)
Badro, J., Côté, A. S. & Brodholt, J. P. A seismologically consistent compositional model of Earth’s core. Proc. Natl Acad. Sci. USA 111, 7542–7545 (2014)
Ozawa, H., Hirose, K., Yonemitsu, K. & Ohishi, Y. High-pressure melting experiments on Fe–Si alloys and implications for silicon as a light element in the core. Earth Planet. Sci. Lett . 456, 47–54 (2016)
Labrosse, S. Thermal evolution of the core with a high thermal conductivity. Phys. Earth Planet. Inter . 247, 36–55 (2015)
Labrosse, S., Hernlund, J. W. & Coltice, N. A crystallizing dense magma ocean at the base of the Earth’s mantle. Nature 450, 866–869 (2007)
Boukaré, C., Ricard, Y. & Fiquet, G. Thermodynamics of the MgO–FeO–SiO2 system up to 140 GPa: application to the crystallization of Earth’s magma ocean. J. Geophys. Res. Solid Earth 120, 6085–6101 (2015)
Buffett, B. A., Garnero, E. J. & Jeanloz, R. Sediments at the top of Earth’s core. Science 290, 1338–1342 (2000)
Hernlund, J. W. & Tackley, P. J. Some dynamical consequences of partial melting in Earth’s deep mantle. Phys. Earth Planet. Inter . 162, 149–163 (2007)
O’Rourke, J. G. & Stevenson, D. J. Powering Earth’s dynamo with magnesium precipitation from the core. Nature 529, 387–389 (2016)
Badro, J., Siebert, J. & Nimmo, F. An early geodynamo driven by exsolution of mantle components from Earth’s core. Nature 536, 326–328 (2016)
Fu, R. R. et al. An ancient core dynamo in asteroid Vesta. Science 338, 238–241 (2012)
Komabayashi, T. Thermodynamics of melting relations in the system Fe–FeO at high pressure: implications for oxygen in the Earth’s core. J. Geophys. Res. Solid Earth 119, (2014)
Christensen, U. R. & Tilgner, A. Power requirement of the geodynamo from ohmic losses in numerical and laboratory dynamos. Nature 429, 169–171 (2004)
Christensen, U. R. Dynamo scaling laws and applications to the planets. Space Sci. Rev . 152, 565–590 (2010)
Fischer, R. A. et al. Phase relations in the Fe–FeSi system at high pressures and temperatures. Earth Planet. Sci. Lett . 373, 54–64 (2013)
Akahama, Y. & Kawamura, H. Pressure calibration of diamond anvil Raman gauge to 310 GPa. J. Appl. Phys. 100, 043516 (2006)
Andrault, D. et al. Thermal pressure in the laser-heated diamond-anvil cell: an X-ray diffraction study. Eur. J. Mineral . 10, 931–940 (1998)
Ichikawa, H., Tsuchiya, T. & Tange, Y. The P-V-T equation of state and thermodynamic properties of liquid iron. J. Geophys. Res. Solid Earth 119, 240–252 (2014)
Morard, G. et al. Melting of Fe–Ni–Si and Fe–Ni–S alloys at megabar pressures: implications for the core–mantle boundary temperature. Phys. Chem. Miner . 38, 767–776 (2011)
O’Neill, H. S. C., Canil, D. & Rubie, D. C. Oxide-metal equilibria at 2500°C and 25 GPa: implications for core formation and the light component in the Earth’s core. J. Geophys. Res. Solid Earth 103, 12239–12260 (1998)
Wade, J. & Wood, B. J. Core formation and the oxidation state of the Earth. Earth Planet. Sci. Lett . 236, 78–95 (2005)
Tsuno, K., Frost, D. J. & Rubie, D. C. Simultaneous partitioning of silicon and oxygen into the Earth’s core during early Earth differentiation. Geophys. Res. Lett . 40, 66–71 (2013)
Ma, Z. Thermodynamic description for concentrated metallic solutions using interaction parameters. Metall. Mater. Trans. B 32, 87–103 (2001)
Japan Society for the Promotion of Science and the Nineteenth Committee on Steelmaking Steelmaking Data Sourcebook Part II (Gordon and Breach, 1988)
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett . 77, 3865–3868 (1996)
Vanderbilt, D. Soft self-consistent pseudopotentials in a generalized eigenvalue formalism. Phys. Rev. B 41, R7892–R7895 (1990)
Giannozzi, P. et al. Quantum ESPRESSO: a modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter 21, 395502 (2009)
Sun, T., Zhang, D. B. & Wentzcovitch, R. M. Dynamic stabilization of cubic CaSiO3 perovskite at high temperatures and pressures from ab initio molecular dynamics. Phys. Rev. B 89, 094109 (2014)
Nosé, S. A unified formulation of the constant temperature molecular dynamics methods. J. Chem. Phys. 81, 511–519 (1984)
Hoover, W. G. Canonical dynamics: equilibrium phase-space distributions. Phys. Rev. A 31, 1695–1697 (1985)
Kuwayama, Y., Hirose, K., Sata, N. & Ohishi, Y. The pyrite-type high-pressure form of silica. Science 309, 923–925 (2005)
González-Cataldo, F., Davis, S. & Gutiérrez, G. Melting curve of SiO2 at multimegabar pressures: implications for gas giants and super-Earths. Sci. Rep . 6, 26537 (2016)
Umemoto, K. et al. Liquid iron-sulfur alloys at outer core conditions by first-principles calculations. Geophys. Res. Lett . 41, 6712–6717 (2014)
Acknowledgements
We thank H. Ozawa, Y. Kidokoro and K. Yonemitsu for their assistance in high high-pressure, high-temperature experiments. Discussions with J. Badro and R. Deguen helped develop the model for core energetics.
Author information
Authors and Affiliations
Contributions
K.H. and G.M. designed the project. K.H., G.M. and R.S. performed experiments, K.U. carried out ab initio calculations, G.H. developed the thermodynamic model, and J.H. and S.L. did the dynamical modelling. The manuscript was written by all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Reviewer Information Nature thanks A. Jephcoat and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Extended data figures and tables
Extended Data Figure 1 TEM bright-field image of the Fe–Si–O starting material.
It consists of fine-grained (<5 nm) metal and oxide.
Extended Data Figure 2 Melting experiment at 133 GPa in run 4.
Radial temperature distribution (as a function of distance from the centre of the laser-heated hot spot) and the composite X-ray maps for Fe, Si, O and Al for a cross-section of the recovered sample; quenched liquid Fe–5.8 wt% Si–0.5 wt% O (green) at the centre, surrounded by solid SiO2 (purple) and unmelted portion (green). They were sandwiched by Al2O3 insulation layers (red). Note that the central part included Al2O3 blocks, which is the evidence that it was molten at high pressure and high temperature.
Extended Data Figure 3 Solubility of O and Si in liquid Fe.
Solid and dashed lines represent  and
and  as parameterized by ref. 9. Points represent experimental data analysed here plotted in various ways; those in red are from this study’s experiments.
as parameterized by ref. 9. Points represent experimental data analysed here plotted in various ways; those in red are from this study’s experiments.  shows an essentially linear 1/T dependence, similar to that found in ref. 35. More thermodynamically correct representation of the solubility K is shown by the
shows an essentially linear 1/T dependence, similar to that found in ref. 35. More thermodynamically correct representation of the solubility K is shown by the  dependence, whose points spread below the
dependence, whose points spread below the  line. This suggests that
line. This suggests that  is largely controlled by
is largely controlled by  , but with additional, minor dependence on
, but with additional, minor dependence on  . When these are accounted for by the modelling procedure described in the text, the result—an effective
. When these are accounted for by the modelling procedure described in the text, the result—an effective  corrected for pressure and non-ideal Si and O interactions in the metal—is shown as
corrected for pressure and non-ideal Si and O interactions in the metal—is shown as  . Grey dotted lines show our model’s
. Grey dotted lines show our model’s  , for comparison.
, for comparison.
Extended Data Figure 4 Solubility limits for SiO2 in liquid Fe superimposed on the present experimental results and earlier metal–silicate partitioning data.
Labelled points show experimental data obtained in this study. Unlabelled points are values used in the model fits5,7,9. No data at pressures <16 GPa are used in the fit, so values below this limit are not well represented by the model. Black curves indicate temperature contours of SiO2 saturation at 135 GPa. Green, blue and red curves are for 2,500 K at 20 GPa, 60 GPa and 100 GPa, respectively.
Extended Data Figure 5 Pressure dependence of SiO2 solubility.
For adiabats initiated at the CMB for a range of present-day core temperatures 3,500 K ≤ TCMB ≤ 4,500 K, we show the combined pressure and temperature dependence of the solubility assuming saturation at the CMB. Each labelled solid line is bounded by the ±2σ confidence interval, showing that within uncertainty, solubility is constant with depth in the core. SiO2 crystallization at the top of the core is a likely scenario, because otherwise crystallization creates compositional stratification, which is inconsistent with seismological observations.
Source data
Rights and permissions
About this article
Cite this article
Hirose, K., Morard, G., Sinmyo, R. et al. Crystallization of silicon dioxide and compositional evolution of the Earth’s core. Nature 543, 99–102 (2017). https://doi.org/10.1038/nature21367
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature21367
This article is cited by
-
Primordial helium extracted from the Earth’s core through magnesium oxide exsolution
Nature Geoscience (2023)
-
Kilometer-scale structure on the core–mantle boundary near Hawaii
Nature Communications (2022)
-
Sustaining Earth’s magnetic dynamo
Nature Reviews Earth & Environment (2022)
-
Melting phase relations in Fe–Si–H at high pressure and implications for Earth’s inner core crystallization
Scientific Reports (2022)
-
Stratification in planetary cores by liquid immiscibility in Fe-S-H
Nature Communications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.