Abstract
Changes in gamma oscillations (20–50 Hz) have been observed in several neurological disorders. However, the relationship between gamma oscillations and cellular pathologies is unclear. Here we show reduced, behaviourally driven gamma oscillations before the onset of plaque formation or cognitive decline in a mouse model of Alzheimer’s disease. Optogenetically driving fast-spiking parvalbumin-positive (FS-PV)-interneurons at gamma (40 Hz), but not other frequencies, reduces levels of amyloid-β (Aβ)1–40 and Aβ 1–42 isoforms. Gene expression profiling revealed induction of genes associated with morphological transformation of microglia, and histological analysis confirmed increased microglia co-localization with Aβ. Subsequently, we designed a non-invasive 40 Hz light-flickering regime that reduced Aβ1–40 and Aβ1–42 levels in the visual cortex of pre-depositing mice and mitigated plaque load in aged, depositing mice. Our findings uncover a previously unappreciated function of gamma rhythms in recruiting both neuronal and glial responses to attenuate Alzheimer’s-disease-associated pathology.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
25 July 2018
In this Article, Extended Data Figure 8 and Extended Data Table 1 contained errors, which have been corrected online.
References
Bartos, M., Vida, I. & Jonas, P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nature Rev. Neurosci. 8, 45–56 (2007)
Fries, P., Nikolić, D. & Singer, W. The gamma cycle. Trends Neurosci. 30, 309–316 (2007)
Cardin, J. A. et al. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature 459, 663–667 (2009)
Palop, J. J. et al. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer’s disease. Neuron 55, 697–711 (2007)
Verret, L. et al. Inhibitory interneuron deficit links altered network activity and cognitive dysfunction in Alzheimer model. Cell 149, 708–721 (2012)
Bero, A. W. et al. Neuronal activity regulates the regional vulnerability to amyloid-β deposition. Nature Neurosci. 14, 750–756 (2011)
Selkoe, D. J. et al. The role of APP processing and trafficking pathways in the formation of amyloid beta-protein. Ann. NY Acad. Sci. 777, 57–64 (1996)
Stam, C. J. et al. Generalized synchronization of MEG recordings in Alzheimer’s disease: evidence for involvement of the gamma band. J. Clin. Neurophysiol. 19, 562–574 (2002)
Gillespie, A. K. et al. Apolipoprotein E4 causes age-dependent disruption of slow gamma oscillations during hippocampal sharp-wave ripples. Neuron 90, 740–751 (2016)
Oakley, H. et al. Intraneuronal β-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: potential factors in amyloid plaque formation. J. Neurosci. 26, 10129–10140 (2006)
Colgin, L. L. et al. Frequency of gamma oscillations routes flow of information in the hippocampus. Nature 462, 353–357 (2009)
Buzsáki, G. Rhythms of the Brain (Oxford Univ. Press, 2006)
Buzsáki, G. et al. Hippocampal network patterns of activity in the mouse. Neuroscience 116, 201–211 (2003)
Carr, M. F., Karlsson, M. P. & Frank, L. M. Transient slow gamma synchrony underlies hippocampal memory replay. Neuron 75, 700–713 (2012)
Foster, D. J. & Wilson, M. A. Reverse replay of behavioural sequences in hippocampal place cells during the awake state. Nature 440, 680–683 (2006)
Carr, M. F., Jadhav, S. P. & Frank, L. M. Hippocampal replay in the awake state: a potential substrate for memory consolidation and retrieval. Nature Neurosci. 14, 147–153 (2011)
Helwig, M. et al. The neuroendocrine protein 7B2 suppresses the aggregation of neurodegenerative disease-related proteins. J. Biol. Chem. 288, 1114–1124 (2013)
Das, U. et al. Activity-induced convergence of APP and BACE-1 in acidic microdomains via an endocytosis-dependent pathway. Neuron 79, 447–460 (2013)
Cataldo, A. M. et al. Endocytic pathway abnormalities precede amyloid beta deposition in sporadic Alzheimer’s disease and Down syndrome: differential effects of APOE genotype and presenilin mutations. Am. J. Pathol. 157, 277–286 (2000)
Gjoneska, E. et al. Conserved epigenomic signals in mice and humans reveal immune basis of Alzheimer’s disease. Nature 518, 365–369 (2015)
Chitu, V. & Stanley, E. R. Colony-stimulating factor-1 in immunity and inflammation. Curr. Opin. Immunol. 18, 39–48 (2006)
Wang, Y. et al. TREM2 lipid sensing sustains the microglial response in an Alzheimer’s disease model. Cell 160, 1061–1071 (2015)
Gray, C. M., König, P., Engel, A. K. & Singer, W. Oscillatory responses in cat visual cortex exhibit inter-columnar synchronization which reflects global stimulus properties. Nature 338, 334–337 (1989)
Eckhorn, R. et al. Coherent oscillations: a mechanism of feature linking in the visual cortex? Multiple electrode and correlation analyses in the cat. Biol. Cybern. 60, 121–130 (1988)
Peričić, D. & Bujas, M. Sex differences in the response to GABA antagonists depend on the route of drug administration. Exp. Brain Res. 115, 187–190 (1997)
Jankowsky, J. L. et al. Mutant presenilins specifically elevate the levels of the 42 residue β-amyloid peptide in vivo: evidence for augmentation of a 42-specific γ secretase. Hum. Mol. Genet. 13, 159–170 (2004)
Chung, K. et al. Structural and molecular interrogation of intact biological systems. Nature 497, 332–337 (2013)
Yoshiyama, Y. et al. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron 53, 337–351 (2007)
Canter, R. G., Penney, J. & Tsai, L.-H. The road to restoring neural circuits for the treatment of Alzheimer’s disease. Nature 539, 187–196 (2016)
Harvey, C. D., Collman, F., Dombeck, D. A. & Tank, D. W. Intracellular dynamics of hippocampal place cells during virtual navigation. Nature 461, 941–946 (2009)
Aronov, D. & Tank, D. W. Engagement of neural circuits underlying 2D spatial navigation in a rodent virtual reality system. Neuron 84, 442–456 (2014)
Rossant, C., et al. Spike sorting for large, dense electrode arrays. Nature Neurosci. 19, 634–642 (2016)
Buzsáki, G. Theta oscillations in the hippocampus. Neuron 33, 325–340 (2002)
Ravassard, P. et al. Multisensory control of hippocampal spatiotemporal selectivity. Science 340, 1342–1346 (2013)
Zhang, Y. et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. 34, 11929–11947 (2014)
Crotti, A. et al. Mutant Huntingtin promotes autonomous microglia activation via myeloid lineage-determining factors. Nature Neurosci. 17, 513–521 (2014)
Erny, D. et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nature Neurosci. 18, 965–977 (2015)
Chiu, I. M. et al. A neurodegeneration-specific gene-expression signature of acutely isolated microglia from an amyotrophic lateral sclerosis mouse model. Cell Reports 4, 385–401 (2013)
Gosselin, D. et al. Environment drives selection and function of enhancers controlling tissue-specific macrophage identities. Cell 159, 1327–1340 (2014)
Huang, S. C.-C. et al. Cell-intrinsic lysosomal lipolysis is essential for alternative activation of macrophages. Nature Immunol. 15, 846–855 (2014)
Yu, H. et al. Tet3 regulates synaptic transmission and homeostatic plasticity via DNA oxidation and repair. Nature Neurosci. 18, 836–843 (2015)
Acknowledgements
We are grateful to S. Tonegawa and D. Roy for APP/PS1 mice, and E. Demmons, W. Raja, E. Wu, and B. Arkhurst and the Boyden laboratory for technical assistance. We thank members of the Tsai and Boyden laboratories, C. Moore, C. Deister, D. Rei, J. Penney, R. Madabhushi, A. Mungenast, A. Bero, and J. Young for discussions and comments on the paper. H.F.I. acknowledges the Cameron Hayden Lord Foundation and Barbara J. Weedon Fellowship; E.S.B. acknowledges the New York Stem Cell Foundation-Robertson Award, National Institutes of Health (NIH) 1R01EY023173, and NIH 1DP1NS087724; L.H.T. acknowledges the JPB Foundation, Belfer Neurodegeneration Consortium, Halis Family Foundation, and NIH RF1 AG047661. E.S.B. and E.N.B. acknowledge NIH ROI GM104948.
Author information
Authors and Affiliations
Contributions
H.F.I, A.C.S., E.N.B, E.S.B., and L.-H.T. designed experiments. H.F.I. and F.G. performed RNA sequencing experiments. H.F.I. and A.C.S. performed electrophysiology. A.C.S. analysed electrophysiology data. H.F.I. performed and analysed optogenetics and ELISA experiments. T.Z.G., J.S., and O.K. performed western blots. H.F.I., A.R., F.A., R.R., and R.G.C. performed and analysed imaging experiments. F.G. analysed RNA sequencing data. H.F.I., A.J.M., and C.A. performed visual stimulation experiments. H.M. performed FACS experiments. H.F.I, A.C.S., A.R., F.G., E.S.B., and L.-H.T. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
L.-H.T. and E.S.B. are scientific founders and serve on the scientific advisory board of Cognito Therapeutics, and H.F.I. and A.C.S. own shares of Cognito Therapeutics Inc.
Extended data figures and tables
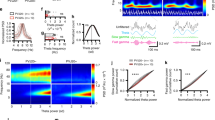
Extended Data Figure 1 5XFAD mice have reduced power in gamma during hippocampal SWRs.
a, Mouse in virtual reality environment. b, LFP recorded in CA1, above, filtered for theta (left) or SWRs (right), middle, and gamma, below. c, Mean and standard deviation of the normalized power spectrum during theta. Each animal’s power spectral density was normalized to its peak (n = 6 mice per group). d, Normalized power spectral densities during theta periods in 3-month-old 5XFAD (green, n = 6 mice) and WT (black/grey, n = 6 mice) mice. Each animal’s power spectral density was normalized to its peak (in theta). e, Average SWR-triggered spectrograms for one WT and one 5XFAD mouse shows an increase in the gamma band during SWRs. This increase is lower in the 5XFAD mouse than in the WT mouse (n = 370 and 514 SWRs in WT and 5XFAD, respectively; WT mouse shown here is the same as in Fig. 1a). This range of frequencies is often called ‘slow gamma’ to distinguish it from faster oscillations (65–140 Hz) that have also been included in the gamma range but for which the origins are less well understood. f, Distributions for each recording (left) and the mean and standard error across sessions (right) of instantaneous gamma frequencies during SWRs in 5XFAD (green) and WT (black) mice show distributions around 40 Hz (n = 820, 800, 679, 38, 1,875, 57 gamma cycles per session in six 5XFAD animals in six recording sessions and 181, 1,075, 919, 1,622, 51, 1,860, 1,903 gamma cycles per session in six WT animals in seven recording sessions). g, Cumulative distribution of the z-scored gamma power during the 100 ms around the peak of the SWR for WT (black) and 5XFAD animals (green) for each animal (left) and the mean and standard error (shaded) across animals (right) (n = 514, 358, 430, 22, 805, 37 SWRs per session in six 5XFAD animals and 82, 311, 370, 776, 18, 710, 818 SWRs per session in six WT animals). h, Fraction of spikes in CA1 during SWRs as a function of the phase of gamma in 5XFAD (green) and WT (black) mice for each animal (left) and the mean and standard error across animals (right, n = 2475, 1060, 3092, 25, 6521, 123 spikes during SWRs per session in six 5XFAD mice and 360, 4741, 1,564, 2,961, 88, 3,058, 4,270 spikes during SWRs per session in six WT mice). i, SWR rate per non-theta period in 5XFAD (green) and WT (black) mice for each animal (left) and all animals combined (right, rank-sum test, P < 10−10, n = 117, 210, 151, 55, 100, 1 non-theta periods per session in six 5XFAD mice and 80, 68, 115, 95, 15, 159, 218 non-theta periods per session in six WT mice). j, The cumulative distribution of gamma power during large SWRs (detection threshold greater than six standard deviations above the mean, Methods) shows significantly smaller increases in 5XFAD (green) than WT (black) mice (rank-sum test, P < 10−5, n = 1,000 SWRs in six 5XFAD mice and 1,467 SWRs in 6 WT mice). k, Fraction of spikes as a function of the phase of gamma during large SWRs (detection threshold greater than six standard deviations above the mean, Methods), mean ± s.e.m. (left) and histogram of the depth of modulation of spiking (right) as a function of gamma phase in 3-month-old 5XFAD (green, n = 6 mice) and WT (black, n = 6 mice) mice (rank-sum test, bootstrap resampling P < 10−10, n = 2500 5XFAD spike-gamma phase distributions and 3,000 WT distributions). l, Power spectral density during 40 Hz stimulation (red, left), random stimulation (blue, centre), or no stimulation (black, right) of FS-PV-interneurons in CA1 for each mouse (n = 4 5XFAD mice with 169, 130, 240, 73 40 Hz, 143, 129, 150, 72 random, and 278, 380, 52, 215 no stimulation periods per animal and n = 3 WT mice with 65, 93, 91 40 Hz, 64, 93, 90 random, and 187, 276, 270 no stimulation periods per animal). m, Above: example raw LFP trace (above) and the trace filtered for spikes (300–6,000 Hz, below), with spikes indicated with red stars after optogenetic stimulation (blue vertical lines). Below: histogram of spikes per pulse after the onset of the 1 ms laser pulse during 40 Hz stimulation (red), random stimulation (blue), or no stimulation (black, n = 345,762 40 Hz, 301559 random pulses, and 32,350 randomly selected no stimulation times at least 500 ms apart from 552 40 Hz, 543 random, and 1681 no stimulation periods in four 5XFAD and three WT mice). n, Histogram of the difference in firing rates between 40 Hz stimulation and random stimulation periods shows that both types of stimulation elicit similar amounts of spiking activity (Wilcoxon signed rank test for zero median, P > 0.6, n = 538 stimulation periods from four 5XFAD and three WT mice, NS, not significant). o, Multiunit firing rates per 40 Hz stimulation (red), random stimulation (blue), and no stimulation (black) period for each animal. Box-and-whisker plots show median (white lines in box) and quartiles (top and bottom of box). In all animals firing rates between 40 Hz and random stimulation were not significantly different, showing that the random stimulation condition serves as a control for spiking activity (rank-sum tests for each animal, three WT and four 5XFAD mice, P > 0.09, n = 87, 130, 8, 65, 93, 91, 73 40 Hz stimulation periods and 85, 129, 5, 64, 93, 90, 72 random stimulation periods per animal). We also examined whether 40 Hz stimulation caused neuronal hyperactivity relative to no stimulation, because, according to a recent report, this could have negative effects on neural circuit function26. In most animals the firing rates between 40 Hz or random stimulation and no stimulation were not significantly different (rank-sum tests for each animal, two WT and two 5XFAD, P > 0.25, n = 8, 93, 91, 73 40 Hz stimulation periods and 15, 277, 270, 215 baseline periods per animal) or the firing rates during 40 Hz or random stimulation were lower than during no stimulation (rank-sum tests for each animal, one WT and one 5XFAD, P < 10−5, which is significant when corrected for performing multiple comparisons, n = 130, 65 40 Hz stimulation periods and 379, 187 baseline periods per animal) indicating that 40 Hz stimulation did not cause neuronal hyperactivity. In one animal there was significantly more activity with 40 Hz or random stimulation than during baseline (rank-sum test for one 5XFAD, mouse, P < 10−5, n = 87 40 Hz stimulation periods and 251 baseline periods per animal). Therefore in six out of seven animals we see no evidence that the 40 Hz optogenetic stimulation of FS-PV-interneurons causes hyperactivity.
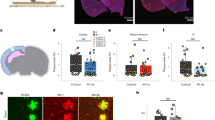
Extended Data Figure 2 ChR2 was expressed in FS-PV-interneurons for optogenetic stimulation.
a, AAV-DIO-ChR2–eYFP or AAV-DIO–eYFP drives Cre-dependent expression of ChR2–eYFP or eYFP to produce cell-type-specific targeting of ChR2 or eYFP, respectively. In the presence of Cre, ChR2–eYFP or eYFP is inverted into the sense direction and expressed from the EF-1α promoter in PV-positive cells. ITR, inverted terminal repeat; polyA; WPRE, woodchuck hepatitis B virus post-transcriptional element. b, ChR2–eYFP was strongly expressed in PV-positive interneurons in CA1 of 3-month-old 5XFAD/PV-Cre mice (scale bar, 100 μm). c, Immunohistochemistry with anti-eYFP and anti-PV antibodies in CA1 of 3-month-old 5XFAD/PV-Cre mice expressing AAV-DIO-ChR2–eYFP shows eYFP expression only in PV-positive cells (scale bar, 50 μm). d, Representative western blots showing levels of full-length APP (top left, CT695), APP CTFs (bottom left, CT695), APP NTF (top right, A8967), and β-actin (bottom right, A5316, loading control) in CA1 in eYFP, random, and 40 Hz stimulation conditions, one mouse per lane, with two biological replicates of each condition. e, Immunohistochemistry with anti-Aβ (12F4, red) antibodies in CA1 of 5XFAD/PV-Cre mice expressing only eYFP or ChR2 with 40 Hz, and random stimulation conditions (scale bar, 50 μm). f, Bar graphs represent the relative immunoreactivity of Aβ normalized to eYFP (n = 4 mice per group; *P < 0.05, ***P < 0.001 by one-way ANOVA). Bar graphs show mean + s.e.m. g, Immunohistochemistry with anti-Rab5 antibody (ADI-KAP-GP006-E, green) in CA1 of 5XFAD/PV-Cre mice (scale bar, 50 μm). h, Relative Rab5 intensity levels normalized to eYFP controls (n = 3 mice per group).
Extended Data Figure 3 Optogenetically driven 40 Hz oscillations in CA1 cause changes in gene regulation and immediate early gene expression.
a, Table of 130 genes upregulated by 40 Hz FS-PV-interneuron stimulation determined by whole-transcriptome RNA-seq of CA1 from 3-month-old 5XFAD/PV-Cre mice (P < 0.05 by Cufflinks 2.2). b, Table of 393 genes downregulated by 40 Hz FS-PV-interneuron stimulation determined by whole transcriptome RNA-seq of CA1 from 3-month-old 5XFAD/PV-Cre mice (P < 0.05 by Cufflinks 2.2). c, Box plot showing FPKM values of up- and downregulated genes in eYFP and 40 Hz groups. Box shows median (black lines in box) and quartiles (top and bottom of box), whiskers show minimum and maximum values, and circles show outliers. d, GSEA statistics tables showing statistical significance of correlation between genes up- or downregulated by 40 Hz stimulation and publicly available neuron-, microglia-, and macrophage-specific RNA-seq data under different chemical and genetic perturbations; the perturbation terms were ranked on the basis of the false discovery rate q values for the upregulated gene list, from the smallest to the largest (Methods). e, RT-qPCR verification of specific gene targets in the RNA-seq dataset. Bar graph shows relative RNA levels (fold change) from eYFP (black) and 40 Hz stimulation (red) conditions (*P < 0.05, **P < 0.01, ***P < 0.001 by Student’s t-test, n = 3 mice per group). All bar graphs show mean + s.e.m. f, Immunohistochemistry with Hoechst to label all cell nuclei in CA1 of 5XFAD/PV-Cre mice expressing only eYFP or ChR2 with 40 Hz stimulation conditions (scale bar, 50 μm). g, Bar graph represents the estimated CA1 thickness in 5XFAD/PV-Cre mice expressing only eYFP or ChR2 with 40 Hz stimulation conditions (n = 4 mice per group; NS, not significant by Student’s t-test).
Extended Data Figure 4 A 40 Hz light flicker drives 40 Hz oscillations in VC, while random flickering does not.
a, Power spectral densities of LFPs in VC during 40 Hz light flicker (red, far left), random light flicker (blue, centre left), dark (black, centre right), or light (green, far right) in VC for each recording session for each mouse (n = 5 recordings from four 5XFAD mice with 47, 51, 64, 49, 16 40 Hz flicker, 47, 50, 64, 50, 16 random flicker, 279, 301, 382, 294, 93 dark and 47, 50, 64, 49, 15 light periods). Light flicker at other frequencies increased power in the flicker frequency, as others have found previously23,24 (data not shown). b, Histogram of the difference in firing rates between 40 Hz light flicker and random light flicker (n = 226 stimulation periods from five recording sessions in four 5XFAD mice). c, Multiunit firing rates in VC during 40 Hz light flicker (red), random light flicker (blue), dark (black), or light (green) periods. Box plots show median (white lines in box) and quartiles (top and bottom of box). In all animals, firing rates between 40 Hz flicker and random flicker conditions were not significantly different showing that the random stimulation condition serves as a control for spiking activity (rank-sum tests for each of five recording session from four 5XFAD mice, P > 0.06, n = 47, 51, 64, 49, 16 40 Hz flicker periods and 47, 50, 64, 50, 16 random flicker periods per recording). There were no significant differences in firing rates between 40 Hz flicker and light conditions, indicating that 40 Hz light flicker generally did not cause neuronal hyperexcitability (rank-sum tests for each of five recording sessions from four 5XFAD mice, P > 0.2 for four recording sessions, P < 0.01 for one recording session, which is not significant when corrected for performing multiple comparisons, n = 47, 51, 64, 49, 16 40 Hz periods and 47, 50, 64, 49, 16 light periods per recording). In one session, there was more activity in the 40 Hz flicker than in the dark condition. d, Example traces of LFPs recorded above the brain during light flicker (above, yellow indicates light on, black indicates light off), during three different recording sessions. e, Power spectral densities of LFPs recorded above the brain during 40 Hz light flicker show no increase in power at 40 Hz. Thus, the effect is not due to photoelectric effects on recording equipment or electrical noise (n = 4, 2, 1, 1, 17, 42, 36, 55, 53 40 Hz flicker periods from four recording sessions in three 5XFAD mice undergoing VC recordings and from five recording sessions in two 5XFAD and three WT mice undergoing hippocampal recordings). Mean (solid line) and standard deviation (shaded area) across recordings are shown on the left and per animal on the right. Recordings with fewer than three flicker periods (light red) resulted in noisier power spectral densities than recordings with more data (dark red) but none showed evidence of peaks at 40 Hz.
Extended Data Figure 5 A 40 Hz light flicker does not affect Aβ levels in hippocampus or barrel cortex.
a, Example LFP trace in hippocampal CA1 before and during 40 Hz light flicker (above). Mean (solid line) and standard deviation (shaded area) of power spectral density during 40 Hz light flicker (red), random light flicker (blue), or dark (black) in CA1 (n = 2 5XFAD and 3 WT mice). b, Histogram of the fraction of spikes in hippocampus as a function of time for four cycles of 40 Hz light flicker (left, red) or the equivalent period of time for random light flicker (right, blue, n = 2 5XFAD and 3 WT mice, mean ± s.e.m. across animals). Bar above indicates when light was on (yellow) or off (black). For random stimulation, spiking was aligned to the start of the light turning on; additional periods with light-on occurred at random intervals are indicated by grey (Methods). c, Histogram of the difference in firing rates between 40 Hz light flicker and random light flicker (bottom n = 168 stimulation periods from five recording sessions in two 5XFAD and three WT mice). d, Power spectral densities of LFPs in CA1 during 40 Hz light flicker (red, far left), random light flicker (blue, centre left), dark (black, centre right), or light (green, far right) for each recording session for each mouse (n = 5 recordings from two 5XFAD and three WT mice with 22, 54, 42, 71, 55, 40 Hz flicker, 12, 34, 32, 54, 36 random flicker, 115, 240, 224, 342, 282 dark and 12, 33, 33, 54, 35 light periods). e, Multiunit firing rates in CA1 during 40 Hz light flicker (red), random light flicker (blue), dark (black), or light (green) periods. Box plots show median (white lines in box) and quartiles (top and bottom of box). In all animals firing rates between 40 Hz flicker and random flicker conditions were not significantly different, showing that the random stimulation condition serves as a control for spiking activity (rank-sum tests for each of five recordings from two 5XFAD and three WT animals, P > 0.2, n = 22, 54, 42, 71, 55 40 Hz flicker periods and 12, 34, 32, 54, 36 random flicker periods per recording). There were no significant differences in firing rates between 40 Hz flicker and light conditions indicating that 40 Hz light flicker generally did not cause neuronal hyperexcitability (rank-sum tests for each of five recordings from two 5XFAD and three WT animals, P > 0.3, n = 22, 54, 42, 71, 55 40 Hz periods and 12, 34, 33, 54, 35 light periods per recording). f, Bar graphs of relative Aβ1–40 levels in VC of 5XFAD mice in dark, 40 Hz flicker, and random flicker conditions, normalized to dark (n = 4 mice per group; NS, not significant). Bars represent mean + s.e.m. Circles superimposed on bars in bar graphs indicate individual data points in each group. g, Bar graphs of relative Aβ1–42 levels in VC of 5XFAD mice in dark, 40 Hz flicker, and random flicker conditions, normalized to dark (n = 4 mice per group; NS indicates not significant). Bars represent mean + s.e.m. Circles superimposed on bars indicate individual data points in each group. h, Bar graph of relative Aβ1–40 and Aβ1–42 levels in barrel cortex of 5XFAD mice in dark and 40 Hz flicker conditions, normalized to dark (n = 3 mice per group; NS, not significant by Student’s t-test).
Extended Data Figure 6 Acute reduction in Aβ after light flicker in APP/PS1 and WT mice.
a, Bar graph of relative Aβ1–40 and Aβ1–42 levels of APP/PS1 in VC in dark and 40 Hz flicker conditions, normalized to dark (n = 4 mice per group for dark and n = 4 mice per group for 40 Hz flicker conditions; NS, not significant, *P < 0.05, by Student’s t-test). All bar graphs show mean + s.e.m. throughout this figure. Circles superimposed on bars in bar graphs indicate individual data points in each group. b, Bar graph of relative mouse Aβ1–40 and Aβ1–42 levels in VC of 9-month-old WT mice in dark and 40 Hz flicker conditions, normalized to dark (n = 11 mice per group for dark and n = 9 mice per group for 40 Hz flicker conditions; *P < 0.05, by Student’s t-test).
Extended Data Figure 7 A 40 Hz light flicker does not decrease synaptic density in VC.
a, Schematic depicting isolation of microglia from VC. VC was dissected, then single cells were suspended and labelled with CD11b and CD45 antibodies. Subsequently, cells were sorted by FACS and lysed. Aβ1–40 levels were analysed by ELISA. b, Bar graph of Aβ1–40 levels in microglia purified using FACS (Methods) from VC of 3-month-old 5XFAD and WT mice (n = 8 mice per group for 5XFAD and n = 4 mice per group for WT mice; *P < 0.05 by Student’s t-test). Circles superimposed on bars indicate individual data points in each group. c, Immunohistochemistry with SVP38 (red) antibodies to detect synaptophysin in VC of 3-month-old 5XFAD mice in dark and 40 Hz flicker conditions (images were taken with 40× objective; scale bar, 50 μm). Right: 100× rendering of dark and 40 Hz flicker conditions. d, Bar graph of relative SVP38 intensity levels in VC of 5XFAD mice after dark (black) and 40 Hz (red) flicker conditions, normalized to dark (n = 4 mice per group; NS, not significant by Student’s t-test).
Extended Data Figure 8 Aβ levels in VC return to baseline 24 h after 1 h of 40 Hz light flicker.
a, Bar graph of relative Aβ1–40 and Aβ1–42 levels in VC of 5XFAD mice 1, 4, 12, and 24 h after 1 h of dark or 40 Hz flicker treatment, normalized to dark (n = 4 mice per group for 4 and 12 h wait, n = 6 for 1 and 24 h wait, n = 12 for dark; NS, not significant, *P < 0.05, **P < 0.01 by one-way ANOVA).
Extended Data Figure 9 Driving 40 Hz oscillations in VC via light flicker reduces phosphorylated tau in a tauopathy mouse model.
a, Immunohistochemistry with anti-pTau (S202, green) and anti-MAP2 (red) antibodies in VC of 4-month-old P301S mice after 7 days of 1 h per day dark or 40 Hz flicker conditions (images were taken with 40× objective; scale bar, 50 μm). Insets: 100× rendering of representative cell body in dark and 40 Hz flicker conditions. No changes were observed by western blot (data not shown). b, Bar graph of relative pTau (S202) intensity levels in P301S mice after 7 days of 1 h per day dark (black) and 40 Hz flicker (red) conditions (n = 8 mice per group; *P < 0.05 by Student’s t-test). All bar graphs show mean + s.e.m. throughout. c, Bar graph of relative MAP2 intensity levels in P301S mice after 7 days of 1 h per day dark (black) and 40 Hz flicker (red) conditions (n = 8 mice per group; NS, not significant by Student’s t-test). d, Immunohistochemistry with anti-pTau (S396, red) antibodies in P301S mice after 7 days of 1 h per day dark and 40 Hz flicker conditions (scale bar, 50 μm). e, Bar graph of relative pTau (S396) fluorescence intensity levels in P301S mice after 7 days of 1 h per day dark (black) and 40 Hz flicker (red) conditions (n = 8 mice per group; ****P < 0.0001 by Student’s t-test). f, Immunohistochemistry with anti-pTau (S404, green) antibodies in P301S mice after 7 days of 1 h per day dark and 40 Hz flicker conditions (scale bar, 50 μm). g, Bar graph of relative pTau (S400/T403/S404) fluorescence intensity levels in P301S mice after 7 days of 1 h per day dark (black) and 40 Hz flicker (red) conditions (n = 8 mice per group; **P < 0.01 by Student’s t-test). Bar graphs show mean + s.e.m. h, Immunohistochemistry with anti-Iba1 (019-19741, green) antibodies in 4-month-old P301S mice after 7 days of 1 h per day dark and 40 Hz flicker conditions (images were taken with 40× objective; scale bar, 50 μm.) Insets: 100× rendering of representative microglia in eYFP and 40 Hz stimulation conditions. i, Bar graph of the number of microglia in P301S mice after 7 days of 1 h per day dark (black) and 40 Hz flicker (red) conditions (n = 8 mice per group; NS, not significant by Student’s t-test). j, Bar graph of the diameter of microglia cell bodies in P301S mice normalized to dark after 7 days of 1 h per day dark (black) and 40 Hz flicker (red) conditions (n = 8 mice per group; ****P < 0.0001 by Student’s t-test). k, Bar graph of the average length of microglia primary processes in P301S mice normalized to control after 7 days of 1 h per day dark (black) and 40 Hz flicker (red) conditions (n = 8 mice per group; ****P < 0.0001 by Student’s t-test).
Supplementary information
40 Hz optogenetic stimulation causes microglia morphological transformation
3D rendering of Iba1-positive microglia and Aβ in hippocampal CA1 region of 5XFAD/PV-Cre mice expressing only EYFP. Immunohistochemistry was performed with anti-Iba1 (019-19741, green) and anti-Aβ (12F4, red) antibodies. Images were taken with 40x objective. (MOV 10054 kb)
40 Hz optogenetic stimulation causes microglia morphological transformation
3D rendering of Iba1-positive microglia and Aβ in hippocampal CA1 region of 5XFAD/PV-Cre mice after 40 Hz stimulation. Immunohistochemistry was performed with anti-Iba1 (019-19741, green) and anti-Aβ (12F4, red) antibodies. Images were taken with 40x objective. (MOV 16012 kb)
40 Hz optogenetic stimulation causes microglia morphological transformation
3D rendering of Iba1-positive microglia and Aβ in hippocampal CA1 region of 5XFAD/PV-Cre mice after random stimulation. Immunohistochemistry was performed with anti-Iba1 (019-19741, green) and anti-Aβ (12F4, red) antibodies. Images were taken with 40x objective. (MOV 11295 kb)
Mouse undergoes light flicker behavioral paradigm
Video of a mouse exposed to light flicker. Video shows 20 Hz because the frame rate obscures the on/off dynamics of 40 Hz light flicker. (MOV 3679 kb)
40 Hz light flicker causes microglia morphological transformation
3D rendering of Iba1-positive microglia after dark condition from CLARITY treated 100 μm tissue sections. Immunohistochemistry was performed with anti-Iba1 (019-19741, green) in 5XFAD visual cortex. Images were taken with 63x objective. (MOV 23572 kb)
Hz light flicker causes microglia morphological transformation
3D rendering of Iba1-positive microglia after 40 Hz flicker from CLARITY treated 100 μm tissue sections. Immunohistochemistry was performed with anti-Iba1 (019-19741, green) in 5XFAD visual cortex. Images were taken with 63x objective. (MOV 27637 kb)
Rights and permissions
About this article
Cite this article
Iaccarino, H., Singer, A., Martorell, A. et al. Gamma frequency entrainment attenuates amyloid load and modifies microglia. Nature 540, 230–235 (2016). https://doi.org/10.1038/nature20587
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature20587
This article is cited by
-
Dopamine neuron degeneration in the Ventral Tegmental Area causes hippocampal hyperexcitability in experimental Alzheimer’s Disease
Molecular Psychiatry (2024)
-
Synchronized neuronal activity drives waste fluid flow
Nature (2024)
-
Neuronal dynamics direct cerebrospinal fluid perfusion and brain clearance
Nature (2024)
-
Brain stimulation with 40 Hz heterochromatic flicker extended beyond red, green, and blue
Scientific Reports (2024)
-
Cognitive and Neuropathophysiological Outcomes of Gamma-tACS in Dementia: A Systematic Review
Neuropsychology Review (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.