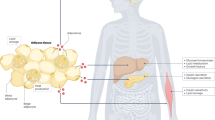
Abstract
Obesity, insulin resistance and the metabolic syndrome are associated with changes to the gut microbiota; however, the mechanism by which modifications to the gut microbiota might lead to these conditions is unknown. Here we show that increased production of acetate by an altered gut microbiota in rodents leads to activation of the parasympathetic nervous system, which, in turn, promotes increased glucose-stimulated insulin secretion, increased ghrelin secretion, hyperphagia, obesity and related sequelae. Together, these findings identify increased acetate production resulting from a nutrient–gut microbiota interaction and subsequent parasympathetic activation as possible therapeutic targets for obesity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
Primary accessions
European Nucleotide Archive
Data deposits
Sequence data are deposited to the European Nucleotide Archive with accession code PRJEB13505.
Change history
28 June 2016
The Reviewer Information section was included.
References
Rahat-Rozenbloom, S., Fernandes, J., Gloor, G. B. & Wolever, T. M. Evidence for greater production of colonic short-chain fatty acids in overweight than lean humans. Int. J. Obes. 38, 1525–1531 (2014)
Shepherd, M. L., Ponder, M. A., Burk, A. O., Milton, S. C. & Swecker, W. S. Jr. Fibre digestibility, abundance of faecal bacteria and plasma acetate concentrations in overweight adult mares. J. Nutr. Sci. 3, e10 (2014)
Turnbaugh, P. J. et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444, 1027–1031 (2006)
Fernandes, J., Su, W., Rahat-Rozenbloom, S., Wolever, T. M. & Comelli, E. M. Adiposity, gut microbiota and faecal short chain fatty acids are linked in adult humans. Nutr. Diabetes 4, e121 (2014)
Li, M. et al. Gut carbohydrate metabolism instead of fat metabolism regulated by gut microbes mediates high-fat diet-induced obesity. Benef. Microbes 5, 335–344 (2014)
Murugesan, S. et al. Study of the diversity and short-chain fatty acids production by the bacterial community in overweight and obese Mexican children. Eur. J. Clin. Microbiol. Inf. Dis. 34, 1337–1346 (2015)
Murphy, E. F. et al. Composition and energy harvesting capacity of the gut microbiota: relationship to diet, obesity and time in mouse models. Gut 59, 1635–1642 (2010)
David, L. A. et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 505, 559–563 (2014)
La-ongkham, O., Nakphaichit, M., Leelavatcharamas, V., Keawsompong, S. & Nitisinprasert, S. Distinct gut microbiota of healthy children from two different geographic regions of Thailand. Arch. Microbiol. 197, 561–573 (2015)
Aguirre, M., Jonkers, D. M., Troost, F. J., Roeselers, G. & Venema, K. In vitro characterization of the impact of different substrates on metabolite production, energy extraction and composition of gut microbiota from lean and obese subjects. PLoS One 9, e113864 (2014)
Shen, W. et al. Protective effects of R-alpha-lipoic acid and acetyl-l-carnitine in MIN6 and isolated rat islet cells chronically exposed to oleic acid. J. Cell. Biochem. 104, 1232–1243 (2008)
Drucker, D. J. Minireview: the glucagon-like peptides. Endocrinology 142, 521–527 (2001)
MacDonald, P. E. et al. The multiple actions of GLP-1 on the process of glucose-stimulated insulin secretion. Diabetes 51 (suppl. 3), S434–S442 (2002)
Ahrén, B. Autonomic regulation of islet hormone secretion—implications for health and disease. Diabetologia 43, 393–410 (2000)
Ronnebaum, S. M. et al. Chronic suppression of acetyl-CoA carboxylase 1 in β-cells impairs insulin secretion via inhibition of glucose rather than lipid metabolism. J. Biol. Chem. 283, 14248–14256 (2008)
Ionescu, E., Rohner-Jeanrenaud, F., Berthoud, H. R. & Jeanrenaud, B. Increases in plasma insulin levels in response to electrical stimulation of the dorsal motor nucleus of the vagus nerve. Endocrinology 112, 904–910 (1983)
Sakaguchi, T. & Yamaguchi, K. Effects of electrical stimulation of the hepatic vagus nerve on the plasma insulin concentration in the rat. Brain Res. 164, 314–316 (1979)
Lee, K. C. & Miller, R. E. The hepatic vagus nerve and the neural regulation of insulin secretion. Endocrinology 117, 307–314 (1985)
Frohman, L. A., Ezdinli, E. Z. & Javid, R. Effect of vagotomy and vagal stimulation on insulin secretion. Diabetes 16, 443–448 (1967)
Bergman, R. N. & Miller, R. E. Direct enhancement of insulin secretion by vagal stimulation of the isolated pancreas. Am. J. Physiol. 225, 481–486 (1973)
Ahrén, B. & Holst, J. J. The cephalic insulin response to meal ingestion in humans is dependent on both cholinergic and noncholinergic mechanisms and is important for postprandial glycemia. Diabetes 50, 1030–1038 (2001)
Yamazaki, H., Philbrick, W., Zawalich, K. C. & Zawalich, W. S. Acute and chronic effects of glucose and carbachol on insulin secretion and phospholipase C activation: studies with diazoxide and atropine. Am. J. Physiol. Endocrinol. Metab. 290, E26–E33 (2006)
D’Alessio, D. A., Kieffer, T. J., Taborsky, G. J., Jr & Havel, P. J. Activation of the parasympathetic nervous system is necessary for normal meal-induced insulin secretion in rhesus macaques. J. Clin. Endocrinol. Metab. 86, 1253–1259 (2001)
Wichmann, A. et al. Microbial modulation of energy availability in the colon regulates intestinal transit. Cell Host Microbe 14, 582–590 (2013)
Acknowledgements
We thank J. Dong, Y. Nozaki, W. Zhu, X. Zhao, J. Stack, M. Kahn, B. Lim, and Y. Kosover for technical assistance. This study was funded by grants from the National Institutes of Health (R01 DK-40936, R01 AG-23686, P30 DK-45735, U24 DK-59635, T32 DK-101019, R01 DK-92606, R01 GM-103574 and DP2 GM-105456) and the Novo Nordisk Foundation Center for Basic Metabolic Research, University of Copenhagen.
Author information
Authors and Affiliations
Contributions
R.J.P., L.P., N.A.B., G.W.C., D.Z., and R.L.C. performed the in vivo and in vitro studies, and all authors analysed data. G.W.C., K.F.P., R.G.K., A.L.G., and G.I.S. provided critical advice for the experiments. Studies were designed and the manuscript was written by R.J.P. and G.I.S. with input from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Reviewer Information Nature thanks D. Piomelli, C. Wollheim and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Extended data figures and tables
Extended Data Figure 2 HFD-fed rats exhibit increased gut acetate production.
a, Plasma triglycerides. b, HOMA-IR. c, Dietary acetate concentrations. n = 2 replicates per diet. d, Faecal acetate normalized to dry weight. e–g, Plasma propionate, whole-body propionate turnover, and faecal propionate concentrations. h–j, Plasma butyrate, whole-body butyrate turnover, and faecal butyrate concentrations. k, [13C]acetate enrichment in plasma of rats fed [13C]bicarbonate-labelled food and water. l, [U-13C]acetate from faeces incubated in [U-13C]glucose or fatty acids. m, In vitro acetate production rate from faeces incubated in [U-13C]glucose or fatty acids. n, In vitro acetate production rate in control, boiled, and UV-irradiated faecal samples. ****P < 0.0001 versus control. o, In vitro faecal acetate production following treatment with antibiotics. Unless otherwise specified, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 versus chow-fed rats; §§P < 0.01 versus 3-day HFD-fed rats by one-way ANOVA with Bonferroni’s multiple comparisons test. In k, data were compared by two-tailed unpaired Student’s t-test. In l–o, data are the mean ± s.e.m. of n = 4 per group, with comparisons to controls via two-tailed unpaired Student’s t-test (n). Unless otherwise specified, n = 6 replicates per group.
Extended Data Figure 3 HFD-fed rats exhibit increased GSIS driven by increased acetate turnover.
a, b, Plasma glucose and glucose infusion rate during a hyperglycaemic clamp. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 versus chow-fed rats. c, Plasma insulin area under the curve (AUC) during the hyperglycaemic clamp. d, Plasma acetate. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 versus 2 μmol kg−1 min−1 acetate; §§§§P < 0.0001 versus 8 μmol kg−1 min−1 acetate. e, f, Plasma glucose and glucose infusion rate during a hyperglycaemic clamp. g, Plasma insulin AUC during the clamp. h, i, Plasma butyrate and whole-body butyrate turnover. *P < 0.05, ****P < 0.0001. j, k, Plasma glucose and glucose infusion rate during a hyperglycaemic clamp. l, m, Plasma insulin concentrations during the hyperglycaemic clamp, and plasma insulin AUC. In all panels, data are the mean ± s.e.m. of n = 6 animals per group, with comparisons by one-way ANOVA with Bonferroni’s multiple comparisons test (a–g) or two-tailed unpaired Student’s t-test (h–m).
Extended Data Figure 4 Increasing total caloric intake leads to increased acetate turnover and GSIS via the microbiota in rats.
a, b, Plasma acetate and whole-body acetate turnover. c, d, Plasma glucose and glucose infusion rate during a hyperglycaemic clamp. e, f, Plasma insulin and insulin AUC during the clamp. g, Caloric intake from protein, fat, and carbohydrate. In g–m, each group was compared to pair-fed, high-carbohydrate-fed rats. h, i, Plasma glucose and glucose infusion rate in the hyperglycaemic clamp. j, k, Plasma acetate and whole-body acetate turnover. l, m, Plasma insulin and insulin AUC during the hyperglycaemic clamp. n, Linear regression: whole-body acetate turnover versus total caloric intake in each diet group. o, p, Plasma glucose and glucose infusion rate during a hyperglycaemic clamp in 4-week HFD-fed rats treated with broad-spectrum non-absorbable antibiotics. q, Plasma acetate. r, Plasma [13C]acetate enrichment following three days of feeding [13C]bicarbonate food and water. Data were compared using the two-tailed unpaired Student’s t-test. s, Insulin AUC during a hyperglycaemic clamp. In all panels, data are the mean ± s.e.m. of n = 6 rats per group, with groups compared by one-way ANOVA with Bonferroni’s multiple comparisons test, unless otherwise stated. In a–f, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 versus 12-h starved rats; §P < 0.05, §§P < 0.01, §§§P < 0.001, §§§§P < 0.0001 versus 48-h starved rats. In h–m, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 versus pair-fed rats given the high-carbohydrate diet. In o–s, ***P < 0.001, ****P < 0.0001 versus HFD-fed rats; §§§P < 0.001, §§§§P < 0.0001 versus antibiotics-treated rats.
Extended Data Figure 5 Faecal transplantation alters recipient microbiomes to resemble their donors as revealed by culture-independent 16S rRNA sequencing of faecal microbiomes from donors and recipients.
a, Relative abundance at the phylum level. Only phyla with relative abundance ≥ 0.1% in at least one group are shown. Data are the mean ± s.e.m. of n = 7–8 replicates per group; *P < 0.05 by 2-tailed unpaired Student’s t-test. b–f, Beta diversity analysis of faecal microbiomes before and after transplantation. The largest component of variation (captured by principal coordinate (PC)1) is shown in b and PC1–PC3 are shown in c–f. Rats from independent litters were randomized before diet administration or faecal transplantation. Beta diversity reflects principal coordinates analysis based on Hellinger distances; the results from unweighted, non-phylogenetic distance metrics and from phylogenetic metrics (weighted and unweighted UniFrac) are similar.
Extended Data Figure 6 The gut microbiota drive increased acetate turnover and GSIS.
a, b, Plasma glucose and glucose infusion rate during a hyperglycaemic clamp in rats following faecal transplant replicates acetate turnover and GSIS in the donor group. c, Plasma acetate. d, Faecal acetate concentration. n = 7 (HFD to chow) or 8 (chow to chow, chow to HFD) per group. e, Plasma insulin AUC. f, Glucose-stimulated insulin release in isolated islets incubated with 400 μM acetate in a physiological buffer. n = 4 per group. g, Plasma C2 acetylcarnitine content. h, Glucose-stimulated insulin release in isolated islets incubated with 100 μM acetylcarnitine. i–m, Plasma alanine, leucine, arginine, glucagon, and GLP-1 concentrations. n, o, Plasma glucose and glucose infusion rate during a hyperglycaemic clamp in acetate-infused rats treated with a GLP-1 inhibitor. p, q, Plasma acetate and whole-body acetate turnover. r, s, Plasma insulin and insulin AUC during the clamp. In all panels, data are mean ± s.e.m. of n = 6 per group. In a–e, ****P < 0.0001 versus chow-fed donor to chow-fed recipient transplants; §§§§P < 0.0001 versus chow-fed donor to HFD-fed recipient transplants by one-way ANOVA with Bonferroni’s multiple comparisons test. Data are the mean ± s.e.m. of n = 6 (HFD to chow) or 7 (chow to chow, chow to HFD) per group. In g–m, *P < 0.05, **P < 0.01 versus 2 μmol kg−1 min−1 acetate; §P < 0.05 versus 8 μmol kg−1 min−1 acetate by one-way ANOVA with Bonferroni’s multiple comparisons test. In g–s, data are the mean ± s.e.m. of n = 6 (unless otherwise specified) per group. In n–s, no significant differences were measured by the two-tailed unpaired Student’s t-test.
Extended Data Figure 7 Acetate drives GSIS via a CNS mechanism.
a, Body weight before and after vagotomy. b, c, Plasma glucose and glucose infusion rate during a hyperglycaemic clamp. d, e, Plasma acetate and whole-body acetate turnover. f, Insulin AUC during the clamp. g, Plasma gastrin during the clamp. h, Plasma glucagon after 120 min of the clamp. h, i, Plasma glucose and glucose infusion rate during a hyperglycaemic clamp in acetate-infused, atropine-treated rats. k, l, Plasma acetate and whole-body acetate turnover. m, Plasma insulin AUC during the clamp. n, Plasma glucagon. In all panels, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 by the two-tailed unpaired Student’s t-test; data represent the mean ± s.e.m. of n = 6 replicates per group.
Extended Data Figure 8 Acetate drives GSIS via parasympathetic activation.
a, b, Plasma glucose and glucose infusion rate during a hyperglycaemic clamp in rats treated with ICV acetate. c, Plasma insulin AUC. d, Plasma glucagon. e, f, Plasma acetate and whole-body acetate turnover in rats treated with systemic intra-arterial acetate and ICV methylatropine. g, h, Plasma glucose and glucose infusion rate during a hyperglycaemic clamp. i, Plasma insulin AUC during the clamp. j, k, Plasma and brain tissue acetate in rats given an injection of acetate into the nucleus tractus solitarius. l, m, Plasma glucose and glucose infusion rate during a hyperglycaemic clamp. n, Plasma insulin AUC during the clamp. o, Plasma glucagon. In all panels, data are the mean ± s.e.m. of n = 6 animals per group, with comparisons by one-way ANOVA with Bonferroni’s multiple comparisons test (a–i) or two-tailed unpaired Student’s t-test (j–o). In b–d, **P < 0.01, ***P < 0.001 versus controls; §§P < 0.01, §§§P < 0.001 versus ICV acetate-treated rats by one-way ANOVA with Bonferroni’s multiple comparisons test. In e–i, ***P < 0.001, ****P < 0.0001 versus controls; §§§P < 0.001, §§§§P < 0.0001 versus acetate-infused rats.
Extended Data Figure 9 Chronic intragastric acetate infusion causes hyperphagia and metabolic syndrome through parasympathetic activation.
a, b, Plasma acetate and whole-body acetate turnover. c, d, Plasma glucose and insulin concentrations during an intraperitoneal glucose tolerance test. e, Insulin AUC during the glucose tolerance test. f, g, Plasma glucose and glucose infusion rate during a hyperglycaemic clamp. h, Insulin AUC during the hyperglycaemic clamp. i, Body weight before and after the infusion study (n = 16 controls, 16 acetate-infused, and 12 acetate-infused and vagotomised rats). j, Caloric intake during the 10-day acetate infusion study. k, Homeostatic model assessment of insulin resistance (HOMA-IR). l, Plasma triglyceride concentrations. m, Plasma insulin at the 120-min time point of a hyperinsulinaemic–euglycaemic clamp. n, o, Plasma glucose and glucose infusion rate during the hyperinsulinaemic–euglycaemic clamp. p, Plasma glucagon. Unless otherwise specified, data are mean ± s.e.m. of n = 6 rats per group, with comparisons by one-way ANOVA with Bonferroni’s multiple comparisons test. In all panels, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 versus controls; §P < 0.05, §§P < 0.01, §§§P < 0.001, §§§§P < 0.0001 versus intragastric acetate-infused rats.
Extended Data Figure 10 Germ-free mice have negligible endogenous short-chain fatty acid production.
a, Ratio of tissue:plasma [13C]acetate in mice fed [13C]bicarbonate. b, c, Plasma and tissue propionate concentrations. d, Plasma [13C]propionate enrichment. e, f, Plasma and tissue butyrate. g, Plasma [13C]butyrate enrichment. h, i, Liver and muscle diacylglycerol concentrations. In all panels, data are the mean ± s.e.m. of n = 9 (GF) or n = 10 (CONV-D) mice per group, with comparisons by two-tailed unpaired Student’s t-test. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 versus CONV-D mice on the same diet.
Supplementary information
Supplementary Information
This file contains Supplementary Methods and additional references. (PDF 296 kb)
Rights and permissions
About this article
Cite this article
Perry, R., Peng, L., Barry, N. et al. Acetate mediates a microbiome–brain–β-cell axis to promote metabolic syndrome. Nature 534, 213–217 (2016). https://doi.org/10.1038/nature18309
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature18309
This article is cited by
-
Microbes little helpers and suppliers for therapeutic asthma approaches
Respiratory Research (2024)
-
Comparison of the efficacy of fish oil and probiotic supplementation on glucose and lipid metabolism in patients with type 2 diabetes: a systematic review and network meta-analysis
Diabetology & Metabolic Syndrome (2024)
-
Efficacy of Bifidobacterium animalis subsp. lactis BL-99 in the treatment of functional dyspepsia: a randomized placebo-controlled clinical trial
Nature Communications (2024)
-
Inter-organ crosstalk during development and progression of type 2 diabetes mellitus
Nature Reviews Endocrinology (2024)
-
Molecular Mechanism of Polysaccharides Extracted from Chinese Medicine Targeting Gut Microbiota for Promoting Health
Chinese Journal of Integrative Medicine (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.