Abstract
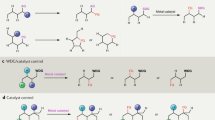
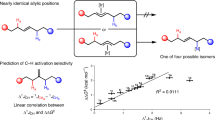
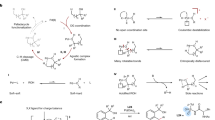
The laboratory synthesis of complex organic molecules relies heavily on the introduction and manipulation of functional groups, such as carbon–oxygen or carbon–halogen bonds; carbon–hydrogen bonds are far less reactive and harder to functionalize selectively. The idea of C–H functionalization, in which C–H bonds are modified at will instead of the functional groups, represents a paradigm shift in the standard logic of organic synthesis1,2,3. For this approach to be generally useful, effective strategies for site-selective C–H functionalization need to be developed. The most practical solutions to the site-selectivity problem rely on either intramolecular reactions4 or the use of directing groups within the substrate5,6,7,8. A challenging, but potentially more flexible approach, would be to use catalyst control to determine which site in a particular substrate would be functionalized9,10,11. Here we describe the use of dirhodium catalysts to achieve highly site-selective, diastereoselective and enantioselective C–H functionalization of n-alkanes and terminally substituted n-alkyl compounds. The reactions proceed in high yield, and functional groups such as halides, silanes and esters are compatible with this chemistry. These studies demonstrate that high site selectivity is possible in C–H functionalization reactions without the need for a directing or anchoring group present in the molecule.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
Data deposits
The crystal data have been deposited in the The Cambridge Crystallographic Data Centre (http://www.ccdc.cam.ac.uk) under accession number 1445448.
References
Yamaguchi, J., Yamaguchi, A. D. & Itami, K. C–H bond functionalization: emerging synthetic tools for natural products and pharmaceuticals. Angew. Chem. Int. Ed. 51, 8960–9009 (2012)
Gutekunst, W. R. & Baran, P. S. C–H functionalization logic in total synthesis. Chem. Soc. Rev. 40, 1976–1991 (2011)
Hartwig, J. F. Evolution of C–H bond functionalization from methane to methodology. J. Am. Chem. Soc. 138, 2–24 (2016)
Doyle, M. P., Duffy, R., Ratnikov, M. & Zhou, L. Catalytic carbene insertion into C–H bonds. Chem. Rev. 110, 704–724 (2010)
Zhang, F. & Spring, D. R. Arene C–H functionalisation using a removable/modifiable or a traceless directing group strategy. Chem. Soc. Rev. 43, 6906–6919 (2014)
Topczewski, J. J. & Sanford, M. S. Carbon–hydrogen (C–H) bond activation at PdIV: a frontier in C–H functionalization catalysis. Chem. Sci. 6, 70–76 (2015)
Engle, K. M., Mei, T.-S., Wasa, M. & Yu, J.-Q. Weak coordination as a powerful means for developing broadly useful C–H functionalization reactions. Acc. Chem. Res. 45, 788–802 (2012)
Colby, D. A., Bergman, R. G. & Ellman, J. A. Rhodium-catalyzed C–C bond formation via heteroatom-directed C–H bond activation. Chem. Rev. 110, 624–655 (2010)
Caballero, A. et al. Catalytic functionalization of low reactive C(sp3)-H and C(sp2)-H bonds of alkanes and arenes by carbene transfer from diazo compounds. Dalton Trans. 44, 20295–20307 (2015)
Kuhl, N., Hopkinson, M. N., Wencel-Delord, J. & Glorius, F. Beyond directing groups: transition-metal-catalyzed C–H activation of simple arenes. Angew. Chem. Int. Ed. 51, 10236–10254 (2012)
Mkhalid, I. A. I., Barnard, J. H., Marder, T. B., Murphy, J. M. & Hartwig, J. F. C–H activation for the construction of C–B bonds. Chem. Rev. 110, 890–931 (2010)
Qin, C. M. & Davies, H. M. L. Role of sterically demanding chiral dirhodium catalysts in site-selective C–H functionalization of activated primary C–H bonds. J. Am. Chem. Soc. 136, 9792–9796 (2014)
Guptill, D. M. & Davies, H. M. L. 2,2,2-Trichloroethyl aryldiazoacetates as robust reagents for the enantioselective C–H functionalization of methyl ethers. J. Am. Chem. Soc. 136, 17718–17721 (2014)
Davies, H. M. L. & Morton, D. Guiding principles for site selective and stereoselective intermolecular C–H functionalization by donor/acceptor rhodium carbenes. Chem. Soc. Rev. 40, 1857–1869 (2011)
Demonceau, A., Noels, A. F., Hubert, A. J. & Teyssie, P. Transition-metal-catalyzed reactions of diazoesters—insertion into C–H bonds of parafins by carbenoids. J. Chem. Soc. Chem. Commun. 14, 688–689 (1981)
Davies, H. M. L., Hansen, T. & Churchill, M. R. Catalytic asymmetric C–H activation of alkanes and tetrahydrofuran. J. Am. Chem. Soc. 122, 3063–3070 (2000)
Thu, H.-Y. et al. Highly selective metal catalysts for intermolecular carbenoid insertion into primary C–H bonds and enantioselective C–C bond formation. Angew. Chem. Int. Ed. 47, 9747–9751 (2008)
Hansen, J. & Davies, H. M. L. High symmetry dirhodium(II) paddlewheel complexes as chiral catalysts. Coord. Chem. Rev. 252, 545–555 (2008)
Davies, H. M. L., Bruzinski, P. R., Lake, D. H., Kong, N. & Fall, M. J. Asymmetric cyclopropanations by rhodium(II) N-(arylsulfonyl)prolinate catalyzed decomposition of vinyldiazomethanes in the presence of alkenes. Practical enantioselective synthesis of the four stereoisomers of 2-phenylcyclopropan-1-amino acid. J. Am. Chem. Soc. 118, 6897–6907 (1996)
Qin, C. M. et al. D2-symmetric dirhodium catalyst derived from a 1,2,2-triarylcyclopropanecarboxylate ligand: design, synthesis and application. J. Am. Chem. Soc. 133, 19198–19204 (2011)
DeAngelis, A., Dmitrenko, O., Yap, G. P. A. & Fox, J. M. Chiral crown conformation of Rh2(S-PTTL)4: Enantioselective cyclopropanation with α-alkyl-α-diazoesters. J. Am. Chem. Soc. 131, 7230–7231 (2009)
Lindsay, V. N. G., Lin, W. & Charette, A. B. Experimental evidence for the all-up reactive conformation of chiral rhodium(II) carboxylate catalysts: enantioselective synthesis of cis-cyclopropane α-amino acids. J. Am. Chem. Soc. 131, 16383–16385 (2009)
Hansen, J., Autschbach, J. & Davies, H. M. L. Computational study on the selectivity of donor/acceptor-substituted rhodium carbenoids. J. Org. Chem. 74, 6555–6563 (2009)
Acknowledgements
Financial support was provided by the National Science Foundation (NSF) through the CCI Center for Selective C–H Functionalization (CHE-1205646). We thank Novartis and AbbVie for supporting our research in C–H functionalization. We thank D. Guptill for conducting some preliminary studies on this project. D.G.M. gratefully acknowledges an NSF MRI-R2 grant (CHE-0958205) and the use of the resources of the Cherry Emerson Center for Scientific Computation.
Author information
Authors and Affiliations
Contributions
K.L. performed and analysed the majority of the synthetic experiments. S.N. prepared the first meta-disubstituted catalyst, D.G.M. conducted the computational studies and J.B. conducted the X-ray crystallographic studies. K.L. and H.M.L.D. designed the synthetic experiments and prepared the manuscript.
Corresponding author
Ethics declarations
Competing interests
H.M.L.D. is a named inventor on a patent entitled “Dirhodium Catalyst Compositions and Synthetic Processes Related Thereto” (US 8,975,428, issued 10 March 2015). The other authors have no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Text and Data – see contents page for details. (PDF 11804 kb)
Rights and permissions
About this article
Cite this article
Liao, K., Negretti, S., Musaev, D. et al. Site-selective and stereoselective functionalization of unactivated C–H bonds. Nature 533, 230–234 (2016). https://doi.org/10.1038/nature17651
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature17651
This article is cited by
-
Tunable molecular editing of indoles with fluoroalkyl carbenes
Nature Chemistry (2024)
-
Bifunctionality of dirhodium tetracarboxylates in metallaphotocatalysis
Nature Communications (2023)
-
Stepwise on-demand functionalization of multihydrosilanes enabled by a hydrogen-atom-transfer photocatalyst based on eosin Y
Nature Chemistry (2023)
-
Visible light-triggered selective C(sp2)-H/C(sp3)-H coupling of benzenes with aliphatic hydrocarbons
Nature Communications (2023)
-
Photochemical diversification of strong C(sp3)–H bonds enabled by allyl bromide and sodium fluoride
Nature Synthesis (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.